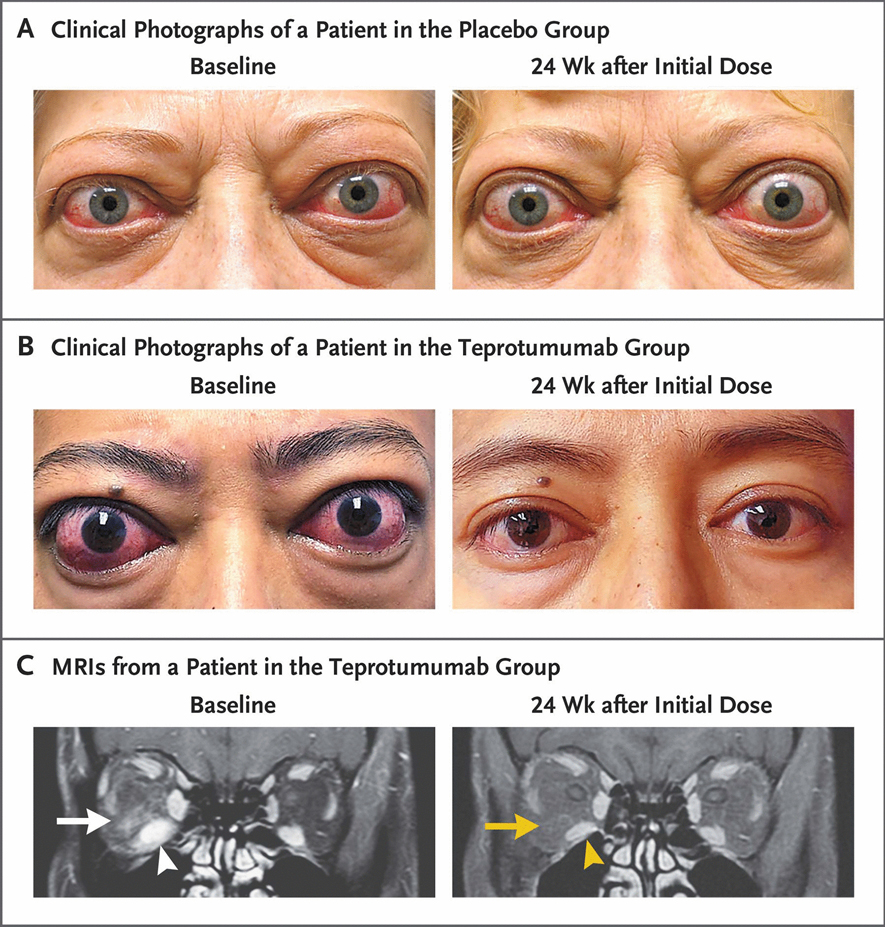
Figure 5.
Phase III trial facial appearance and orbital MRIs at baseline and 24 weeks after initial saline or teprotumumab infusion. (a) Frontal view of a patient receiving placebo. At baseline, considerable bilateral proptosis (OD, 29 mm and OS, 27 mm) and inflammatory signs (Clinical Activity Score 7 OD and 5 OS). Proptosis and inflammation persist at week 24. (b) Frontal view of a patient receiving teprotumumab. At baseline, proptosis (24 mm OU), edema, upper and lower eyelid retraction, and multiple inflammatory signs (Clinical activity score 5 OU) are present. Reductions of proptosis of 5 mm OU and reduction of CAS 4 points OU observed at 24 weeks (c) T1-weighted coronal and contrast-enhanced, magnetic resonance images at baseline and week 24 in a patient treated with teprotumumab. Enhancement of inferior rectus muscle (OD, white arrowhead) and adipose tissue (white arrow) reflect findings of inflammation and edema. The respective inferior rectus muscle (yellow arrowhead) and orbital fat are shown at week 24 (yellow arrow). The inferior rectus volume was reduced by 49% by Materialise software (yellow arrowhead). Proptosis reduction decreased 5 mm from baseline at week 24. Reprinted with permission from Douglas RS et al N Engl J Med. 2020; copyright 2020 Massachusetts Medical Society