Abstract
Plasma medicine is a rapidly expanding field that utilizes non-equilibrium plasma discharges at atmospheric conditions or in liquids for clinical applications. There is significant interest in the production of plasma in the liquid phase for wastewater treatment, agricultural applications, and medical purposes. However, little investigation has been done about the effects of dielectric coatings on submerged electrodes, which is of significant interest to limit electrical current flow in the liquid. This work investigates the effect of different dielectric coatings including aluminum oxide, parylene C, and bi-layer combinations, on plasma discharge characteristics in phosphate-buffered saline (σ = 18 mS/cm) from nanosecond high-voltage pulses. Observed results for aluminum oxide are consistent with past works, including micron-sized clusters of holes generated in the layer due to dielectric breakdown. A bi-layer combination of parylene C on top of aluminum oxide resulted in longer lifetime for electrodes, possibly due to the melting/solidification behavior of the polymer, which may have a “healing” effect. The use of a thick parylene C layer resulted in a different, “creeping”, discharge regime, which is hypothesized to be similar to triple-gap discharge observed in space plasma physics and high-voltage insulators, in which the electric field is enhanced at the boundary of a conductor, dielectric, and a vacuum/fluid, resulting in discharge at this junction point. Temporally-resolved and high-spatial-resolution imaging are required for verification.
I. INTRODUCTION
Non-equilibrium plasmas generated in liquids are being investigated for many potential applications. The removal of organics including phenols and dyes [1], [2], [3], [4], [5], [6], [7], [8]; treatment of bacteria and biofilms [4], [7], [9], [10], [11], [12], [13], [14]; water softening [15], [16]; and materials applications such as nanoparticle synthesis and surface functionalization [8], [17] have been demonstrated.
Plasma generation in conducting liquids is of interest for multiple applications including plasma medicine, wastewater treatment, and others, but results in challenging issues. Bare metal electrodes in conductive liquids lead to significant current flow through the bulk liquid, dissipating power via Joule heating instead of being directed toward plasma generation. For repetitively-pulsed systems this can result in liquid heating over time followed by phase change, which simplifies plasma generation, but bubble formation may be an unwanted side effect, particularly in biomedical applications.
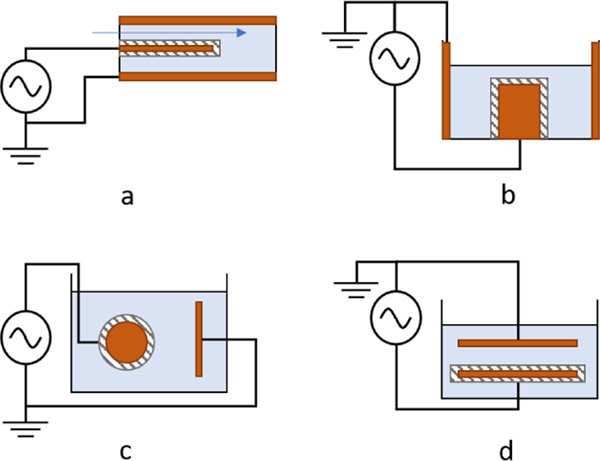
Dielectric coatings have been investigated as a method to limit direct current flow between electrodes in conductive liquids and to increase discharge intensity. Sunka et al. explored dielectric coatings on electrodes for plasmas in liquids [18]. A porous-quartz-ceramic coating was deposited onto a pin electrode for a liquid feed-through reactor, shown schematically in Figure 1a, and operated using microsecond-scale discharges of up to 50 kV applied voltage. The coating provided additional streamer discharges along the length of the pin, in contrast to the localized discharge at the electrode tip observed for bare metal electrodes. The authors suggest an electric field enhancement at the dielectric–dielectric interface between the ceramic coating and the liquid, in addition to local field enhancements due to charge build-up at pores and imperfections along the ceramic surface.
Fig. 1.
Various dielectric-coated-electrode configurations: (a) coaxial feed-through reactor with dielectric-coated wire anode; (b) coaxial reactor with dielectric-coated cylindrical anode; (c) dielectric-coated spherical anode to plate cathode reactor; (d) dielectric-coated plate to bare-metal plate reactor.
These results were later utilized by Lukes et al., who created cylindrical, ceramic-coated electrodes for discharge in conductive liquids, depicted in Figure 1b [19], [20], [21]. Streamers were longer and more localized at regions of high electric field strength at low conductivity (< 1 mS/cm), whereas streamers were smaller and more uniformly distributed across the device at higher conductivity (1.5, 15 mS/cm). The dielectric layer was sufficiently thick (0.2–0.3 mm) such that the layer was not broken down; however, no imaging was done to evaluate changes to surface morphology due to plasma processes.
Zhu et al. created streamer discharges in conductive liquid using a dielectric-coated-spherical-electrode-to-conductive-plate-electrode configuration depicted schematically in Figure 1c [22], [23]. Streamer discharge was observed at varying voltages and liquid conductivities, all resulting in channels through the dielectric coating. These channels, or pores, in the dielectric coating were formed via dielectric breakdown of the material. Plasma is preferentially formed within the existing channels in subsequent pulses, which might be analogous to capillary plasma devices that initiate plasma in bubbles formed by localized Joule heating.
Sein et al. deposited a 50 μm porous aluminum-oxide (Al2O3) layer via thermal plasma spraying onto the cathode of a plate-to-plate reactor, depicted in Figure 1d [24]. A 25 kV pulse of 300-ns pulse width (typical) was used to generate plasma in liquid with conductivity ≈ 1–3 μS/cm. The creation of pores in the dielectric layer due to dielectric breakdown of the Al2O3 was observed through scanning electron microscopy. These pores then provided a conducting channel between the electrodes, increasing the current and lowering the discharge intensity of the device, indicating that continued pore formation was detrimental to sustained operation.
This study provides new insight about nanosecond-scale plasma discharge in high conductivity liquids with dielectric coatings for future applications where limiting current flow in the liquid is required, particularly in plasma medicine. An Al2O3 dielectric layer on one electrode of a pin-to-rod configuration, as well as the biocompatible polymer, parylene C, and bi-layer combination of the two, in a high conductivity liquid, phosphate-buffered saline (PBS), are investigated. The goal of our larger project is to develop electrode designs that provide long lifetime of dielectric coatings in conducting liquids, many 10’s of millions of pulses, which would be required in future clinical applications. These reported results did not meet our goals, but these observations are of value to the plasma medicine community, particularly as it relates to the use of biocompatible polymers as dielectrics.
Section II discusses the experimental setup and methods used, Section III presents results and discussion for the three needle types including electrical measurements, electron micrographs of needle damage, and optical imaging of the discharge characteristics. Section IV provides hypotheses for the discharge behaviors that are observed and Section V provides a concluding summary.
II. METHODS
The schematic of the experimental setup is shown in Figure 2a, and an image is shown in Figure 2b. A dielectric-coated tungsten needle with a sharp tip served as the anode and the cathode was a blunted bare-tungsten rod of 750 μm diameter. Electrodes were tested in a modified, polystyrene semi-micro cuvette. Symmetric holes were drilled into the narrow sides to allow for electrode access, then sealed with silicone after electrode alignment. Electrodes were separated with an approximately 5-mm gap. The vessel was filled with 400 μL of PBS for each test with measured conductivity, σ = 18 mS/cm. This was determined by diluting at a 4 : 1 ratio with distilled water and measuring with a handheld electrical conductivity meter.
Fig. 2.
Experimental setup for testing effect of dielectric coatings on discharge in conductive liquid media. Tungsten needles are used as electrodes with the anode coated in different dielectric layers. A blunted, bare tungsten rod electrode acts as the cathode. A polystyrene semi-micro cuvette was modified to allow electrode access from the sides and pipette access from the top. Electrodes were aligned, then sealed with silicone. Electrode spacing is ≈ 5-mm. The system is driven with a floating high-voltage nanosecond pulser. Current is measured with a Pearson current monitor and voltage is measured across a capacitive voltage divider with an active high-voltage differential probe. Optical imaging is synchronized with the pulser using a function generator.
Three different conformal coatings were investigated in this study: (a) ≈ 500-nm-thick Al2O3; (b) Al2O3 (same thickness) and ≈ 3.9 μm parylene C; (c) parylene C-only layers of ≈ 45.8 μm thickness.
Needles used for Al2O3 and bi-layer Al2O3–parylene C electrodes discussed in Sections III-A and III-B were prepared by electrochemically etching 750 μm-diameter tungsten rods in 8% NaOH solution at 3.0-V applied bias. Needles were ultrasonically cleaned in distilled water and rinsed in methanol before a dielectric coating was deposited. Typical tip radius was on the order of 1–10 μm, as measured using field-emission scanning electron microscopy (FESEM).
Atomic layer deposition (ALD) was used to deposit a conformal Al2O3 coating onto the needles. A 508.1-nm layer of Al2O3 was deposited onto the needles using water and trimethylaluminum precursors in an ALD system (Savannah 200). Layer thickness was measured by fitting a Cauchy model to the ellipsometry data of a silicon wafer control sample.
To improve coating performance, Al2O3-coated needles were coated with a second layer comprised of parylene C (ϵr ≈ 3), a bio-accepted polymer with high dielectric strength (6.8 kV/mil). Parylene C, hereafter referred to as parylene, was deposited using an evaporator system (Model PDS 2010 LABCOATER). Parylene film thickness was 3.9 μm, measured via prolifometry of a silicon wafer control sample.
Electrodes reported in Section III-C are commercially-available tungsten needles (Ted Pella Inc., 13570). The commercial tungsten needles exhibited a 520 μm diameter with a tip radius of approximately 1 μm, the bare counter-electrode is the blunt tungsten rod described previously. Observing the results of the Al2O3 and parylene-coated electrodes, these electrodes were coated in 45.8 μm parylene through the same method as above, foregoing Al2O3.
A pulsed potential was applied to the electrodes using a high-voltage pulse generator (Eagle Harbor Technologies, Nanosecond Pulser) that generates a selectable 0.3–30 kV applied voltage and 20–240-ns pulse width, with rise time on the order of nanoseconds. Pulse repetition rate is selectable from single shot to 10 kHz. Voltage was measured using a differential probe (Tektronix, THDP0100) and a capacitive voltage divider of 200-fF total capacitance. Current was measured on the cathode side (low side) using a current monitor (Pearson Electronics Inc., 2100). The voltage probe and current monitor were sampled by a 1-GSPS oscilloscope (Tektronix, DPO2012B).
A visible light camera (Imaging Development Systems Inc., UI-3280CP-C-HQ Rev.2) captured plasma light emissions throughout operation. The camera and high-voltage pulser were triggered by a function generator to initiate the camera exposure 10’s of μs before discharge initiation. Exposure time was fixed at 900 μs, so was much longer than the pulse width to collect all light from the pulse, but short enough that multiple discharges are not integrated. Gain was varied depending on the intensity of the emission.
Electrode surfaces were examined using FESEM, before and after high-voltage operation, to determine discharge effects on dielectric coating conditions, as well as electrode shape and structure.
III. RESULTS
A. Al2O3 Coating
The first dielectric to be investigated was Al2O3. Images of discharge during operation under different voltages (12 kV and 27 kV) at 40-ns pulse width from pulses up 1200 are shown in Figure 3. Images show the plasma light emission at each pulse number (p). At both voltages, multiple discharge sites were observed along the anode (needle). The number of discharge sites was initially higher at 27 kV compared to 12 kV. The emission color is observed to change from purple/orange during the first pulse to dominantly orange during the second pulse. The color orange might be interpreted as the relative amount of sodium (Na+ at 598.0 nm and 598.6 nm) ions excited within the discharge region or as a decrease in black-body temperature, although spectral information is required for verification. Our existing spectrometer was not sensitive enough for measurement. The oxide layer is ideally conformal before discharge; therefore, damage to the dielectric coating via dielectric breakdown may also be responsible for the change in discharge color.
Fig. 3.
Images of discharge of Al2O3-coated tungsten needles operated in PBS at 12 and 27 kV with a 40-ns pulse width. The first pulse is dominantly purple, whereas the subsequent pulses transition to dominantly orange, suggesting either a decrease in blackbody temperature of the streamer or an increase in emission by sodium ions. Images of discharge for pulses p = 30, 120, 420, 1200 show decreasing intensity, as well as number of discharge sites.
Discharge emissions were detected throughout the 1200 pulses at both 12 kV and 27 kV applied voltage, although the number of discharge sites and their emission intensity decreased over time. The 27 kV case began with a greater number of visible discharge locations compared to the 12 kV case, yet ended with fewer locations due to increased anode damage. It is noted here that observations of the bare cathode rod did not show any visible emission with our camera capabilities.
Figure 4 shows the measured current for needles operated at 240-ns pulse width at 12 kV and 27 kV, with pulse repetition frequencies of 2 Hz and 20 Hz. The current waveforms were similar at each voltage and were independent of repetition rate or needle. The waveforms resemble current through a resistive element with an initial charging and ringing from load capacitance. Measurement bandwidth and resolution were not sufficient to identify discharge peaks, and current waveforms of the Al2O3-coated needles strongly resembled the bare-metal case. It is noted here, but not shown, that experiments with both anode and cathode uncoated did not show any observable discharge, demonstrated resistive current waveforms, and rapidly resulted in boiling of the liquid when operated at high repetition rates (several kHz).
Fig. 4.
Current waveforms of the first discharge for tungsten needles coated in 508.1-nm Al2O3, operated at 240-ns pulse width in PBS. Four diffferent needles were utilized at operating voltage of 12 kV and 27 kV and pulse repetition frequencies of 2 Hz and 20 Hz. The needles exhibit resistive behavior similar to bare-metal electrodes, and needle-to-needle variance was small despite differences in tip shape from electrochemical etching.
Needles were examined using FESEM. The conformal nature of the dielectric layer was verified visually before discharge. In the “after” micrographs, significant damage to the oxide layers was observed, whereas minimal change was observed for the underlying tungsten.
Dielectric coating damage manifested in several ways, as shown in Figure 5. In all needles, small, pore-like channels were created through the oxide layer (Figure 5a). Pore diameters were approximately 1–3 μm. In some cases, large portions of the oxide layer delaminated, leaving exposed tungsten at the tip of the needles (Figure 5b,c). The streamer channel diameter during dielectric breakdown of the oxide layer might be estimated to be approximately 1–3 μm based on the observed pore size, and larger areas of damage are suspected to be caused by the accumulation and expansion of pores. The formation of such pores was also observed by Sein et al. for 50 μm Al2O3-ceramic coatings on plate electrodes [24].
Fig. 5.
Electron micrographs of surface damage observed on post-discharge Al2O3-coated anodes via FESEM: (a) clustered pores; (b,c) delamination at the tip. Pores are formed by the dielectric breakdown of the coating, and delamination of larger areas may have arisen due to formation of pore clusters.
The observed decrease in needle lifetime, defined as the number of pulses after which zero emission sites are visible, with increasing voltage and repetition rate can be explained by changes to plasma intensity, duration, and heat exchange at the anode surface. At higher voltages, additional discharge locations occur due to an increase in electric field strength along the anode. An increase in repetition rate decreased the time between pulses, allowing local temperatures to stay warmer than at low repetition rate due to the (relatively) slow diffusion between liquid at the anode surface and the bulk liquid.
B. Al2O3 and Parylene C Bilayer
The Al2O3-parylene bilayer electrodes increased the emission intensity and reduced the number of discharge locations relative to the 508-nm Al2O3 coating alone. Discharges were sufficiently bright for visual inspection during the first several pulses. Example images of discharge are shown in Figure 6a for 27 kV applied voltage, 240-ns pulse width, and 4-Hz repetition rate up to 1200 pulses. The addition of parylene extended anode lifetime, although a similar decrease in emission intensity was observed over the 1200 pulses. Discharge emission was more purple than observed in the Al2O3 needles for the first several dozen pulses, which might indicate additional dielectric breakdown or increased plasma intensity.
Fig. 6.
(a) Images of discharge for different pulse numbers of tungsten needles coated in a 508-nm-Al2O3 and 3.9 μm-parylene bilayer operated at 27 kV applied voltage, 240-ns pulse width, and 4-Hz repetition rate in PBS. Discharge emission was more intense than for Al2O3-coated needles throughout operation, and discharges exhibited a purple color for several pulses before transitioning to orange. FESEM micrographs of the 500 μm-Al2O3 and 3.9 μm-parylene bilayer-coated needles, before (b) and after (c) 1200 pulses. High local plasma temperature resulted in observable melting of the parylene layer.
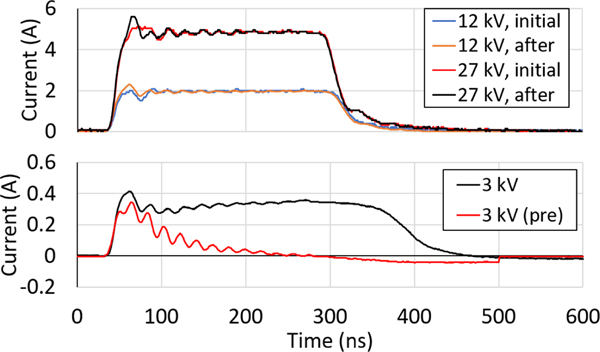
Correspondingly, the addition of a parylene coating changed discharge current behavior during early pulses. The current waveforms of the first pulse and a later pulse (approximately p = 200) during operation at 12 kV and 27 kV are shown in Figure 7 (top). During the first pulse, current is limited by the dielectric layer and does not reach the peak observed in Al2O3-coated needles. Near the first current peak of the first pulse, a fluctuation occurs that might be representative of partial breakdown of the dielectric layer. This is followed by a full (hard) breakdown of the coating and the familiar resisitive behavior indicating the formation of a conductive channel through the coating. After continuous pulsing, capacitive behavior decreases and the current waveform more closely resembles the resistive case observed for the Al2O3-coated needles.
Fig. 7.
(top) Current waveforms of the first and approximately 200th discharge of tungsten needles coated with 508-nm Al2O3 and 3.9 μm parylene, operated in PBS at 12 kV and 27 kV applied voltages, 240-ns pulse width, and 4-Hz repetition rate. Current measurements show fluctuations at the first peak, suggesting dielectric breakdown of the bilayer. Repeated discharge, and associated electrode damage, lessens this behavior until the needles behave resistively. (bottom) Pre- and post-breakdown current waveforms of tungsten needles coated with 508-nm Al2O3 and 3.9 μm parylene, operated at 3 kV in PBS. Only displacement current is observed for the pre-discharge system due to capacitance from the intact dielectric layer. Resistive behavior is observed after breakdown, and capacitive behavior appears during the onset of the pulse despite electrode damage.
The capacitive behavior of the dielectric-coated needle is more observable at lower voltages. Pre-discharge and post-discharge current waveforms are plotted in Figure 7 (bottom). The system behaved as a capacitor before discharge, and only displacement current was observed. After an initial breakdown, the system exhibited more complex behavior. The capacitive behavior is observed at the beginning of the pulse until a sudden increase in current occurred, whereupon the system begins to behave resistively. This behavior indicates that, despite the anode undergoing hard dielectric breakdown, the system still retained some capacitance.
The retention of capacitive behavior might be explained by polymer characteristics. Melting and reformation of the parylene layer can reform streamer-induced pores, such as those shown in FESEM micrographs of Al2O3-coated needles in Figure 5a. This “healing” of the device would limit current flow and encourage additional dielectric-breakdown discharges. However, discharge behavior began to resemble the Al2O3-coated needles after continued pulsing, indicating severe damage to the dielectric layer. These hypotheses are supported by the melting of the needle observed by comparing the FESEM micrographs in Figure 6b (prior to experiments) and Figure 6c (after experiments).
C. Thick Parylene C Coating
Discharge behavior for thicker parylene-only coatings deviated from results for thinner coatings. Discharge occurred only near the tip of the electrode for the first several hundreds of pulses, indicating that electric field strength was sufficient for breakdown only at the anode tip. Electric field enhancement at needle imperfections were no longer sufficient for breakdown along the needle shaft.
FESEM micrographs of parylene-coated tungsten electrodes prior to experiments are shown in Figure 8a. A “dimple” was seen at the tip of each electrode in the pre-discharge images due to charging of the dielectric surface during FESEM, and is not a physical feature. Parylene deposition at the extreme contact angles of the 1 μm-radius tungsten tips appeared conformal, and the final tip radius was consistent with expectations (50 μm).
Fig. 8.
FESEM micrographs of (a) before and (b) after plasma discharge of tungsten needles coated with 45.8 μm parylene operated at 27 kV in PBS. Each needle was operated for 1200 pulses at 240-ns pulse width and 2-Hz repetition rate. Repeated discharge destroyed the parylene coating at each needle tip. The underlying tungsten tip showed a decrease in tip radius and signs of melting.
Parylene was removed from the anode tips after approximately 1200 pulses, exposing bare metal, as shown in Figure 8b. Thin cracks in the parylene coating appeared, stemming from the tip and down the needle shaft. Significant blunting of the metal tips occurred, with final tungsten tip radius near 20–30 μm. Similar blunting was observed by Sunka et al. [18], as a result of melting due to high plasma temperatures. The tips were non-uniformly coated with parylene residue.
The voltage and current waveforms of the first discharge for a parylene-coated tungsten needle at 12 kV applied voltage are plotted in Figure 9a and Figure 9b for 240-ns and 80-ns pulse width, respectively. In Figure 9, the current waveform first resembles capacitive charging in response to an impulse voltage (displacement current). Current begins to decrease as charge is accumulated across the dielectric layer until a threshold voltage is reached. Partial discharge(s) develop as the voltage across the dielectric layer continues to increase, followed by a full breakdown of the dielectric layer. At this point, a conducting plasma channel forms and the system behaves as a resistive element, similar to the bare-metal case. Similar behavior is observed at both 240 ns and 80 ns, although the 80 ns current waveform is affected by the rounded pulse shape (i.e., there is no flattening of the voltage pulse).
Fig. 9.
Voltage and current waveforms of discharge using a tungsten needle coated in 45.8 μm parylene, operated at 12 kV applied voltage for (a,c) 240-ns pulse width and (b) 80-ns pulse width in PBS. Current behavior resembles a capacitor (i) as the pulse voltage rises. Following charging, dielectric breakdown (ii) occurs and a conductive plasma channel is formed (iii). Breakdown occurred at lower voltages on successive pulses (c) due to damage of the parylene layer (240-ns pulse width data shown).
The plasma inception voltage decreased as pulse number increased due to erosion of the parylene layer. From the first to second pulses, a decrease from 19 kV to 15 kV was observed for a 45.8 μm coated tungsten needle, plotted in Figure 9c. This trend is expected to continue until sufficient conductive surface area is exposed on the needle such that the discharge resembles the earlier Al2O3-coated needles due to resistive losses.
Images of the parylene-coated anodes during operation are shown in Figure 10a. Early in the device lifetime, discharge is localized near the tip of the needle. Over several thousand pulses, discharge begins to propagate down the needle shaft. Propagation of the discharge does not occur at new, spontaneous locations, but rather as an expansion from existing discharge locations near dielectric–metal–fluid interfaces, or triple-points. Discharge occasionally occurred at the tip of the needle, regardless of whether or not the parylene coating had been eroded from the area, which is likely due to the increased electric field at this location.
Fig. 10.
(a) Images of discharge in PBS for a tungsten needle with 45.8 μm parylene coating, operated at 27 kV applied voltage and 240-ns pulse width, over approximately 5 minutes. Plasma first appears at the anode tip. Discharge then appears near the dielectric-metal interface, and propagates down the anode shaft. Discharge intermittently appears at the anode tip despite damage to the dielectric layer. (b) Images of needle condition throughout operation in PBS for a tungsten needle with 45.8 μm parylene coating, operated at 27 kV applied voltage and 240-ns pulse width over approximately 5 minutes. The propagating discharge continues to damage the parylene layer throughout operation. Parylene at the anode tip is eventually delaminated and ejected by shockwaves.
Non-discharge images were also taken immediately after each pulse throughout operation. In Figure 10b, the progression of damage to the dielectric layer is shown. Thin cracks extend across the needle shaft due to discharge progression, removing portions of the parylene layer. At one point in operation, the parylene coating at the tip of the needle is cleaved and removed from the needle by shockwaves. Bubble and shockwave formation were readily observed in all cases.
IV. DISCUSSION OF DISCHARGE BEHAVIOR
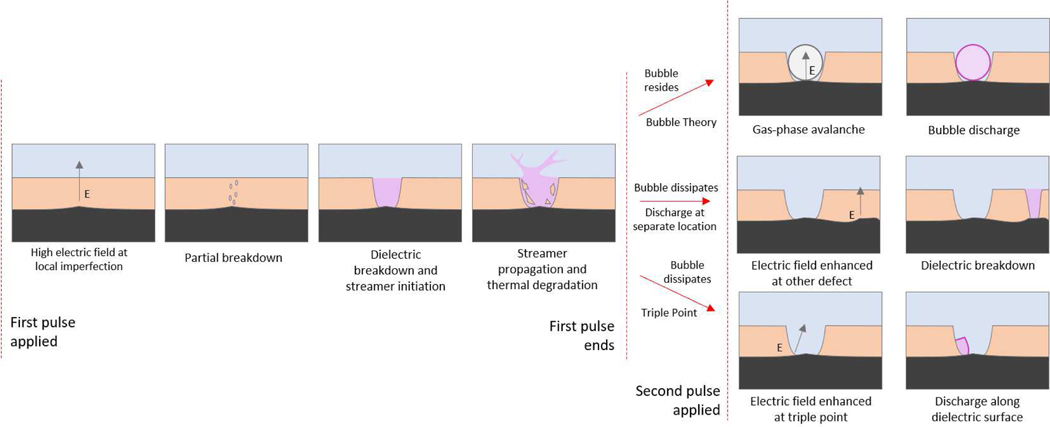
A diagram of the proposed mechanisms for pulse-to-pulse device behavior is shown in Figure 11. The first pulse leads to dielectric breakdown of the dielectric layer. After a conducting plasma channel is formed through the material, the discharge may propagate into the bulk liquid. The high temperature of the discharge is expected to further degrade the dielectric material surrounding the streamer, leading to increased erosion or melting of the surrounding material. This is particularly the case for parylene coatings, which exhibit a melting point under 300 °C, but will also occur with other dielectric coatings.
Fig. 11.
Possible discharge mechanisms for dielectric-coated needles in conductive liquid. (left) shows possible behavior during the first applied pulse. The black layer is the electrode the peach layer is the insulator and the blue layer is the liquid. Local electrode imperfections lead to an enahnced electric field resulting in eventual dielectric breakdown and plasma formation in the pore. Depending on plasma duration, the pore may widen due to thermal degradation. (right) shows possible behavior for second and subsequent applied pulses. The existing pore may be left with a bubble in which plasma may initiate (top); a bubble may not exist or may have dissipated between pulses and other local imperfections may result in dielectric breakdown as before (middle); the conductor–dielectric–liquid junction in the pore may lead to enhanced electric field as in triple-gap junctions leading to breakdown at this location (bottom).
Bubble formation is a known possible consequence of discharges in liquids [25]. Two situations may occur by the arrival of the second pulse: the residence of the bubble left from the gaseous plasma, or the dissipation of the bubble. If the bubble resides, the succeeding discharges may occur due to bubble discharge mechanisms. Bubble discharge is possible at nanosecond timescales due to the bubble existing before the potential is applied, i.e., bubble formation by Joule heating is not required.
If the bubble dissipates, there are two additional methods considered for discharge. First, the existing pores contribute only to resistive losses and a dielectric breakdown occurs at a new location on the anode. This idea is supported by the clusters of discharges observed on the anodes via FESEM as in Figure 5, indicating that numerous discharges occurred within micrometers of each other that might be observed by camera as a single discharge. It is also possible that these discharge locations might have appeared simultaneously from a single pulse. Second, electronic mechanisms that rely on the formation of low-density regions in the liquid exist for discharge within the existing pores [26]. Electrostriction or other mechanisms may lead to discharge near the triple-point formed by the dielectric layer and exposed metal.
A triple-point between a dielectric, conductor, and vacuum/fluid is known to enhance the local electric field and encourage electron avalanche, causing flashover or multipactor discharge, and is a common observation in space plasmas and high-voltage insulation. The enhanced field at the triple-point is considered to possibly lead to an increase in field-emitted electrons from the cathode or to increase secondary electron emission from the dielectric surface near the anode [27], [28]. Investigation of pre-breakdown electron emission in insulated high voltage systems indicates thin dielectric layers or ”islands” covering a metallic electrode may have a combined effect on triple-point emission [29], [30]. These additional electron emission pathways would decrease the voltage required for breakdown, encouraging breakdown along the parylene surface at the anode–parylene–liquid junctions via electronic mechanisms or by discharge in local micro-bubbles.
Continued discharge was observed at the electrode tip after erosion of the dielectric layer. Through FESEM, a thin, non-uniform layer of parylene was observed remaining on the electrodes, despite significant delamination and melting of the tip. Dielectric breakdown of this layer, or additional electric field enhancement at the electrode tip, may have contributed to discharge in this region.
These processes would continue for both the Al2O3-coated and parylene-coated needles. As anode erosion continues, resistive losses limit discharge intensity. Lastly, a notable consequence of this discharge method is release of coating material into the bulk liquid due to melting and vaporization of the dielectric layer.
Further investigation is required using temporally-resolved and high spatial resolution imaging to verify these different behaviors, but is outside the scope of the overall project.
V. CONCLUSIONS
This work discusses the effect of dielectric coatings in phosphate-buffered saline in a pin-to-rod nanosecond pulsed non-equilibrium plasma experiment. Greater understanding of dielectric coatings on electrodes has future applications in plasma medicine, as well as other in-liquid plasma applications such as water treatment. The use of dielectric coatings can reduce driven current through conducting liquids, preventing boiling, which may be undesirable, particularly for plasma medicine applications. The use of biocompatible polymers, such as parylene, is of particular interest in plasma medicine, but the necessity for long lifetime (10’s of millions of pulses) of such systems in clinical settings requires a greater understanding of discharge characteristics and effects on the coatings. This study is the first such investigation.
Conformal coatings of aluminum oxide, parylene C, and a bi-layer combination of the two deposited on tungsten needle electrodes were investigated. Needles with Al2O3 coatings demonstrated a large number of discharge locations along the length of the needle with the number and intensity of these sites reducing significantly as the number of pulses increased, which is evidence of damage to the coatings. This was verified with FESEM showing the generation of pores in the dielectric layer and, in some cases, full delamination of the coating, likely associated with accumulation and aggregation of pore damage. Pore generation has also been reported by other groups under similar circumstances.
Addition of 3.9 μm of parylene reduced the number of discharge locations while increasing the discharge intensity at remaining locations. This additional layer also extended the lifetime of the needles, perhaps due to “self-healing” characteristics of polymers (i.e., melting and then reforming over the damage), which is supported by FESEM micrographs showing melt behavior of the parylene.
Finally, thick coatings (45.8 μm) of parlyene only were investigated. These needles demonstrated new behavior with initial discharge occurring at the needle tip, but then propagating down the length of the needle as the number of pulses increased. The coating was cleaved and eventually removed from the needles. We propose a mechanism to explain these observations: triple-point junctions in which a dielectric, conductor, and another medium, liquid in this case, meet, resulting in an enhancement to the local electric field, leading to discharge at these locations. Triple-point discharges are well-known in space plasma systems and high-voltage insulator systems, but appear to have significance here, as well.
Dielectric coatings will be an important aspect of in-liquid plasma generation systems, particularly those that operate in conducting liquids and seek to limit current flow in these liquids, such as in biomedical applications. Parylene and Al2O3 show promise but, under the operating conditions reported here, result in limited electrode lifetime. More investigation is required into different coating types and different operating regimes to further improve the lifetime and performance of these systems.
ACKNOWLEDGMENTS
This study was partially supported by the National Institutes of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number R21EB024693. Partial support was also provided by NIH Award NCATS TL1TR002016 The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biography
Timothy R. Brubaker, Ph. D. Timothy Brubaker received his Ph.D. degree in Electrical Engineering at The Pennsylvania State University in 2019. At the time of this research, his work focused on optical plasma diagnostics and atmospheric-pressure plasma systems for biomedical applications.

McKayla Nicol McKayla is a graduate student in the school of Veterinary and Biomedical Sciences at The Pennsylvania State University. She is currently pursuing a dual-title PhD in Pathobiology and Clinical and Translational Sciences. McKayla also completed her undergraduate degree at Penn State, double majoring in Biomedical and Mechanical Engineering. McKayla works with the Low-Temperature Plasma Science and Engineering group investigating biomedical applications of low temperature plasmas. Her other areas of focus include characterizing immune responses to bacterial pathogen, Francisella tularensis, with the goal of developing vaccines.

Girish Kirimanjeswara, Ph. D. Girish Kirimanjeswara received D.V.M. degree from Bangalore Veterinary College, Bangalore, India in 1995 and M.V.Sc. in Biotechnology in 1998 from Indian Veterinary Research Institute, Izatnagar, India. He received Ph.D. degree in pathobiology in 2005 from the Pennsylvania State University, University Park, PA. He was a postdoctoral research scholar and research assistant professor in the center for Immunology and Microbial Disease, Albany Medical College, Albany, NY (2005–2011). He is currently an Associate professor of Immunology and infectious Disease at the Dept of Veterinary and Biomedical Sciences, the Pennsylvania State University, University Park, PA. His research includes bacterial pathogenesis and management of drug resistant bacterial infections as well as role of micronutrients in infection and immunity.

Christopher A. Siedlecki, Ph. D. Chris Siedlecki is the Jane A. Fetter Professor of Surgery and a Professor of Biomedical Engineering at the Penn State College of Medicine, Hershey PA. His work focuses on biomaterials, medical devices and blood-material interactions with particular emphasis on hemocompatibility and device-centered infections.
Ali Kazemi Ali Kazemi is a graduate student in the Department of Biomedical Engineering at Penn State. His work has focused on biomedical imaging and the potential of plasma medicine.

Philip C. Snyder Philip Snyder is a senior majoring in Aerospace Engineering and Astronomy and Astrophysics at The Pennsylvania State University. He is a member of the Low-Temperature Plasma Science and Engineering Group and is also involved in the Penn State Pulsar Search Collaboratory and Sigma Gamma Tau aerospace engineering honor society. He is planning to pursue a doctoral degree in aerospace engineering upon graduation.

Sven G. Bilén, Ph. D. Sven G. Bilén (S’90–M’98–SM’08) received the B.S. degree from Pennsylvania State University, University Park, PA, USA, 1991, and the M.S.E. and Ph.D. degrees from the University of Michigan, Ann Arbor, MI, USA, in 1993 and 1998, respectively.
He is a Professor of engineering design, electrical engineering, and aerospace engineering with Pennsylvania State University and the Head of the School of Engineering Design, Technology, and Professional Programs. His current research interests, coordinated through his direction of the Systems Design Laboratory, include the areas of space systems design, electrodynamic tethers, spacecraftplasma interactions, plasma diagnostics for space plasmas, low-temperature plasma physics, plasma electric thrusters, and semiconductor plasma processing, software-defined radio techniques and systems, wireless sensor systems, innovative engineering design, systems design, and new product design, engineering entrepreneurship, and global and virtual engineering design.

Sean D. Knecht, Ph. D. Sean Knecht is an Assistant Teaching Professor in the School of Engineering Design, Technology, and Professional Programs (SEDTAPP) at The Pennsylvania State University and Head of the Low-Temperature Plasma Science and Engineering Group. His work focuses on applications of low-temperature plasmas including biomedical applications. Other areas of investigation include fusion plasmas, aerospace plasmas, and biomedical imaging.
Contributor Information
Timothy R. Brubaker, The Pennsylvania State University
McKayla J. Nicol, The Pennsylvania State University
Girish Kirimanjeswara, The Pennsylvania State University.
Christopher A. Siedlecki, Penn State College of Medicine, Hershey PA
Ali Kazemi, The Pennsylvania State University.
Philip C. Snyder, The Pennsylvania State University
Sven G. Bilén, The Pennsylvania State University.
Sean D. Knecht, The Pennsylvania State University
REFERENCES
- [1].Sharma AK, Locke BR, Arce P, and Finney WC, “A preliminary study of pulsed streamer corona discharge for the degradation of phenol in aqueous solutions,” Hazardous Waste and Hazardous Materials, vol. 10, no. 2, pp. 209–219, 1993. [Google Scholar]
- [2].Sun B, Sato M, and Clements JS, “Use of a pulsed high-voltage discharge for removal of organic compounds in aqueous solution,” Journal of Physics D: Applied Physics, vol. 32, p. 1908, 1999. [Google Scholar]
- [3].Sugiarto AT and Sato M, “Pulsed plasma processing of organic compounds in aqueous solution,” Thin Solid Films, vol. 386, pp. 295–299, 2001. [Google Scholar]
- [4].Locke BR, Sato M, Sunka P, Hoffmann MR, and Chang JS, “Electrohydraulic discharge and nonthermal plasma for water treatment,” Industrial and Engineering Chemistry Research, vol. 45, pp. 882–905, 2006. [Google Scholar]
- [5].Dang TH, Denat A, Lesaint O, and Teissedre G, “Degradation of organic molecules by streamer discharges in water: Coupled electrical and chemical measurements,” Plasma Sources Science and Technology, vol. 17, p. 024013, 2008. [Google Scholar]
- [6].Shi J, Bian W, and Yin X, “Organic contaminants removal by the technique of pulsed high-voltage discharge in water,” Journal of Hazardous Materials, vol. 171, pp. 924–931, 2009. [DOI] [PubMed] [Google Scholar]
- [7].Ruma P. Lukes, Aoki N, Spetlikova E, Hosseini SH, Sakugawa T, and Akiyama H, “Effects of pulse frequency of input power on the physical and chemical properties of pulsed streamer discharge plasmas in water,” Journal of Physics D: Applied Physics, vol. 46, p. 125202, 2013. [Google Scholar]
- [8].Horikoshi S. and Serpone N, “In-liquid plasma: A novel tool in the fabrication of nanomaterials and in the treatment of wastewaters,” RSC Adv., vol. 7, p. 47196, 2017. [Online]. Available: http://xlink.rsc.org/?DOI=C7RA09600C [Google Scholar]
- [9].Zuckerman H, Krasik YE, and Felsteiner J, “Inactivation of microorganisms using pulsed high-current underwater discharges,” Innovative Food Science and Emerging Technologies, vol. 3, pp. 329–336, 2002. [Google Scholar]
- [10].Fudamoto T, Namihira T, Katsuki S, Akiyama H, Imakubo T, and Majima T, “Sterilization of E. coli by underwater pulsed streamer discharges in a continuous flow system,” Electrical Engineering in Japan (English translation of Denki Gakkai Ronbunshi), vol. 164, no. 1, pp. 1–7, 2008. [Google Scholar]
- [11].Gupta SB and Bluhm H, “The potential of pulsed underwater streamer discharges as a disinfection technique,” IEEE Transactions on Plasma Science, vol. 36, no. 4, pp. 1621–1632, 2008. [Google Scholar]
- [12].Li Z, Ohno T, Sato H, Sakugawa T, Akiyama H, Kunitomo S, Sasaki K, Ayukawa M, and Fujiwara H, “A method of water-bloom prevention using underwater pulsed streamer discharge,” Journal of Environmental Science and Health - Part A Toxic/Hazardous Substances and Environmental Engineering, vol. 43, pp. 1209–1214, 2008. [DOI] [PubMed] [Google Scholar]
- [13].Abou-Ghazala A, Katsuki S, Schoenbach KH, Dobbs FC, and Moreira KR, “Bacterial decontamination of water by means of pulsed corona discharges,” IEEE Transactions on Plasma Science, vol. 30, no. 4, pp. 1449–1453, 2002. [Google Scholar]
- [14].Khan MSI, Lee EJ, and Kim YJ, “A submerged dielectric barrier discharge plasma inactivation mechanism of biofilms produced by Escherichia coli O157:H7, Cronobacter sakazakii, and Staphylococcus aureus,” Scientific Reports, vol. 6, p. 37072, 2016. [Online]. Available: 10.1038/srep37072 [DOI] [Google Scholar]
- [15].Yang Y, Fridman A, and Cho YI, Plasma Discharge in Water. Elsevier Inc., 2010, vol. 42. [Online]. Available: 10.1016/S0065-2717(10)42003-1 [DOI] [Google Scholar]
- [16].Yang Y, Kim H, Starikovskiy A, Fridman A, and Cho YI, “Application of pulsed spark discharge for calcium carbonate precipitation in hard water,” Water Research, vol. 44, no. 12, pp. 3659–3668, 2010. [Online]. Available: 10.1016/j.watres.2010.04.024 [DOI] [PubMed] [Google Scholar]
- [17].Saito G. and Akiyama T, “Nanomaterial synthesis using plasma generation in liquid,” Journal of Nanomaterials, vol. 2015, p. 123696, 2015. [Google Scholar]
- [18].Sunka P, Simek M, Clupek M, Schmidt J, and Cern M, “Generation of chemically active species by electrical discharges in water,” Plasma Sources Science and Technology, vol. 8, p. 258, 1999. [Google Scholar]
- [19].Lukes P, Clupek M, Sunka P, and Babicky V, “Effect of ceramic composition on pulse discharge induced processes in water using ceramic-coated wire to cylinder electrode system,” Czechoslovak Journal of Physics, vol. 52, pp. D800–D806, 2002. [Google Scholar]
- [20].Lukes P, Clupek M, Babicky V, and Sunka P, “Pulsed electrical discharge in water generated using porous-ceramic-coated electrodes,” IEEE Transactions on Plasma Science, vol. 36, no. 4, pp. 1146–1147, 2008. [Google Scholar]
- [21].Lukes P, Clupek M, Sunka P, and Babicky V, “The role of surface chemistry at ceramic/electrolyte interfaces in the generation of pulsed corona discharges in water using porous ceramic-coated rod electrodes,” Plasma Processes and Polymers, vol. 6, pp. 719–728, 2009. [Google Scholar]
- [22].Zhu T, Yang L, Jia Z, and Zhang Q, “Characteristics of streamer discharge development between the dielectric-coated sphere-plane electrodes in water,” Journal of Applied Physics, vol. 104, p. 113302, 2008. [Google Scholar]
- [23].Zhu T, Zhang Q, Jia Z, and Yang L, “The effect of conductivity on streamer initiation and propagation between dielectric-coated sphere-plate electrodes in water,” IEEE Transactions on Dielectrics and Electrical Insulation, vol. 16, no. 6, pp. 1552–1557, 2009. [Google Scholar]
- [24].Sein MM, Bin Nasir Z, Telgheder U, and Schmidt TC, “Studies on a non-thermal pulsed corona plasma between two parallel-plate electrodes in water,” Journal of Physics D: Applied Physics, vol. 45, p. 225203, 2012. [Google Scholar]
- [25].Locke BR and Thagard SM, “Analysis and review of chemical reactions and transport processes in pulsed electrical discharge plasma formed directly in liquid water,” Plasma Chemistry and Plasma Processing, vol. 32, pp. 875–917, 2012. [Google Scholar]
- [26].Zhuang J, Sun A, and Huo C, “Formation mechanism of streamer discharges in liquids: A review,” High Voltage, vol. 1, no. 2, pp. 74–80, 2016. [Online]. Available: http://digital-library.theiet.org/content/journals/10.1049/hve.2016.0016 [Google Scholar]
- [27].Anderson RA, “Mechanism of fast surface flashover in vacuum,” Applied Physics Letters, vol. 24, p. 54, 1974. [Google Scholar]
- [28].Jordan N, Lau Y-Y, French DM, and Gilgenbach RM, “Electric field and electron orbits near a triple point,” Journal of Applied Physics, vol. 102, p. 033301, 2007. [Google Scholar]
- [29].Latham R, “The origin of prebreakdown electron emission from vacuum-insulated high voltage electrodes,” Vacuum, vol. 32, pp. 137–140, 1982. [Google Scholar]
- [30].Schachter L, “Analytic expression for triple-point electron emission from an ideal edge,” Appl. Phys. Lett, vol. 72, p. 421, 1998. [Google Scholar]