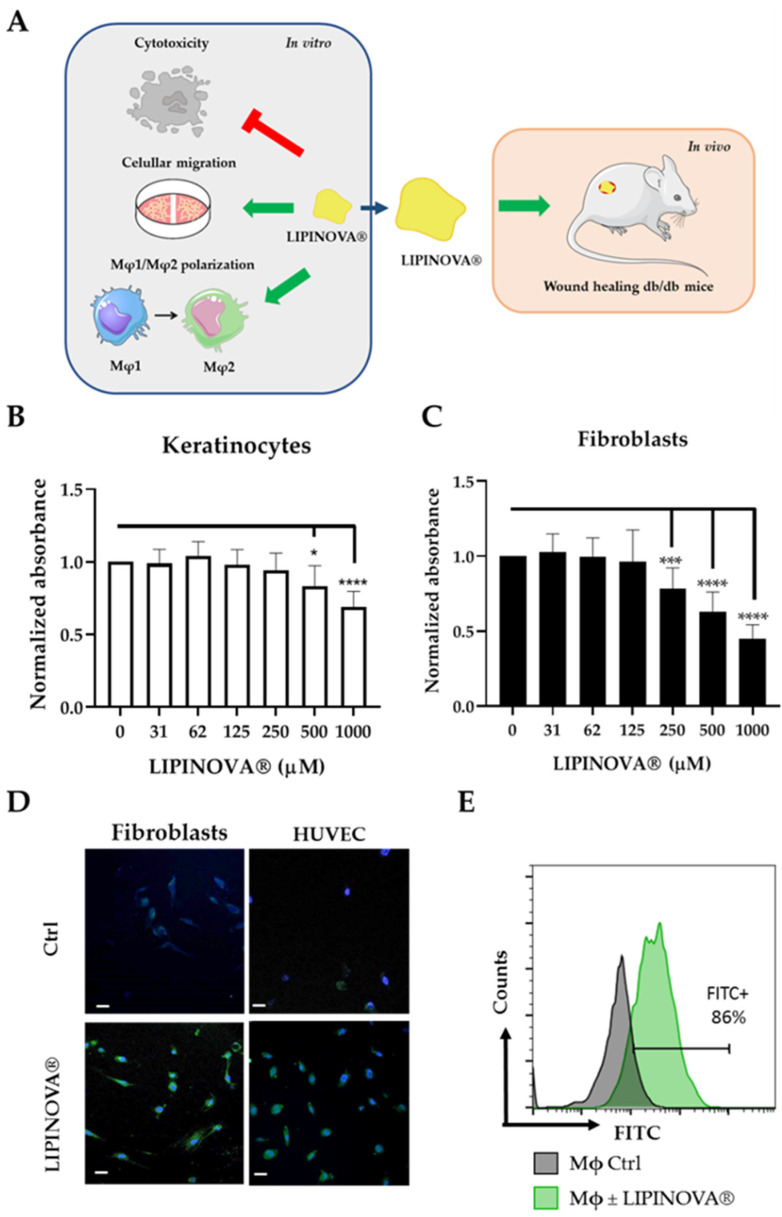
Figure 1.
Keratinocyte and fibroblast viability as a function of LIPINOVA® concentration. (A) Schematic design of the study. LIPINOVA® was assessed in vitro for cytotoxicity; uptake by dermal fibroblasts, endothelial cells, and macrophages; scratch wound healing; and macrophage polarization. Thereafter, the therapeutic potential of LIPINOVA® was measured in an in vivo wound healing assay in db/db mice. Viability of (B) keratinocytes and (C) fibroblasts assessed with the CCK-8 assay after incubation with different concentrations of LIPINOVA®. Absorbance was measured at 450 nm, and data are represented as mean ± SD of three independent experiments. Two-way ANOVA was used for statistical analysis. * p < 0.05, *** p < 0.001, and **** p < 0.0001. (D) Immunofluorescence analysis of HUVEC after 6 h incubation with 100 µM LIPINOVA®-OG or saline (Ctrl). Cells were fixed and stained with DAPI (blue). Images were acquired with a fluorescence microscope with a 20× objective. Scale bar = 50 µm. (E) Flow cytometry assay to measure LIPINOVA® internalization in human macrophages.