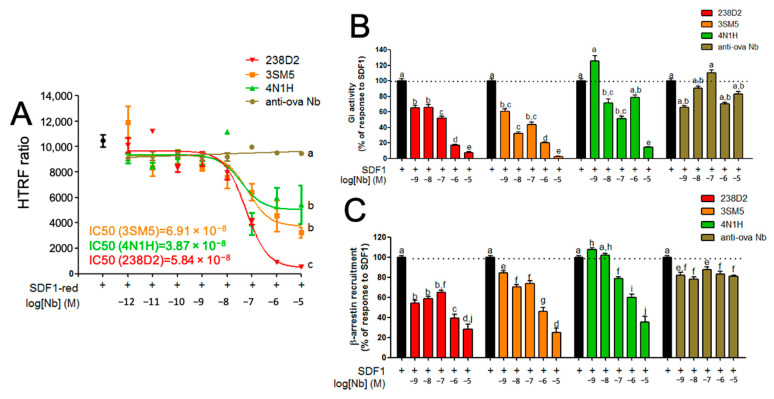
Figure 3.
3SM5 and 4N1H bind CXCR4 and trigger the same intracellular signaling as 238D2. (A) SDF1 displacement. HEK293FT cells expressing the Terbium-linked SNAP-tagged CXCR4 were incubated with d2-coupled SDF1 to measure the ligand binding as an initial FRET signal (black dot). Increasing doses of the Nbs (238D2 in red, 3SM5 in orange, 4N1H in green, and the anti-ovalbumin Nb in brown) from 1 pM to 10 µM were added. Results are shown as the FRET ratio of emission light of the acceptor (d2) divided by the emission of the donor (Tb). (B) Gi protein activity. HEK 293FT cells transiently expressing the hCXCR4 and the CAMYEL cAMP sensor were incubated with forskolin (100 µM) to induce basal cAMP production and SDF1 (50 nM) to induce Gi activation (black bars). The BRET ratio (emission of acceptor/emission of donor) was calculated while increasing Nb concentration from 1 nM to 10 µM. (C) β-arrestin recruitment to CXCR4. In HEK293FT cells transiently expressing Rluc8-fused CXCR4 and YPET-β-arr2, β-arrestin 2 recruitment to the CXCR4 was induced with 50 nM SDF1 (black bars). BRET ratio was measured at indicated concentrations of Nb (from 1 nM to 10 µM). Identical letters show no statistical difference, and different letters show statistical differences at p < 0.05.