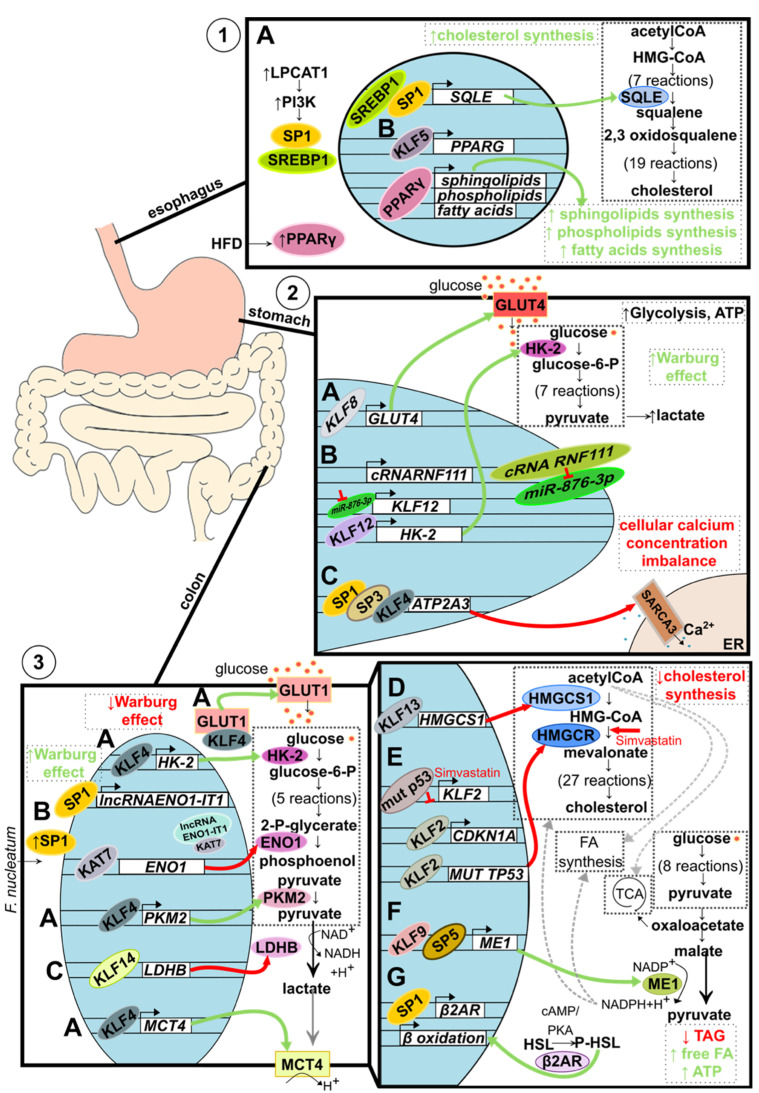
Figure 3.
Metabolic alteration in GI track-associated cancer. (1) Alteration of lipid metabolism in esophageal cancer. (A) Increased level of LPCAT1 activates PI3K signaling pathways, which leads to SP1 and SREBP1 recruitment into the nucleus. Both transcription factors bind to the SQLE regulatory element and induce its expression resulting in increased de novo cholesterol synthesis. (B). Elevated during the tumorigenesis, KLF5 binds to the enhancer and promoter regions of PPARG, activating its expression. PPARγ then binds to the promoters of sphingolipids, phospholipids, and fatty acids synthesis-related genes resulting in increased de novo synthesis. Additionally, the level of PPARγ is stimulated environmentally by a high-fat diet (HFD). (2) Alteration of glucose metabolism in gastric cancer. (A) Upregulated KLF8 binds to the GLUT4 promoter stimulating its expression and, as a consequence, increasing glucose uptake. (B) Interaction between circular RNA RNF111 and its target, miR-876-3p, leads to decreased ability of miR-876-3p to downregulate KLF12 expression. Consequently, upregulated KLF12 stimulates HK-2 expression leading to increased lactate and ATP production. Additionally, KLF12 is believed to positively affect glucose uptake, leading to an increased Warburg effect. (C) SP1, SP3, and KLF4 collectively bind to the ATP2A3 proximal promoter downregulating its expression. Lowered level of sarco/endoplasmic reticulum Ca2+ ATPase SERCA3 results in a loss of intracellular Ca2+ homeostasis and tumorigenesis suppression. (3) Alteration of glucose and lipids metabolism in colorectal cancer. (A) KLF4 decreases the Warburg effect by acting as a tumor suppressor, affecting glucose metabolism on multiple levels. KLF4 binds to the promoter region and upregulates the expression of key glycolytic enzymes: HK-2 and PKM2. Additionally, KLF4 upregulates the expression of lactate transporter MCT4 and stimulates translocation of GLUT1 into the cell membrane. By doing so, KLF4 stimulates the overall glucose uptake and oxidative glucose metabolism and prevents lactic acid buildup. (B) Microbiota component, F. nucleatum, increases intracellular levels of SP1, leading to the induction of SP1-dependent lncRNA-ENO1-IT1 expression and histone acetyltransferase KAT7 recruitment. KAT7 changes the availability of the ENO1 gene, regulates its expression, and downregulates glycolysis. (C) KLF14 binds to the LDHB promoter downregulating its expression. (A–C) Taken together, KLFs and SPs, in the case of colorectal cancer, act as tumor suppressors by turning glucose metabolism into less Warburg effect-like. (D) KLF13 binds to the HMGCS1 promoter and downregulates its expression resulting in decreased de novo cholesterol synthesis. (E) Similarly, KLF2 reduces de novo cholesterol synthesis by mediating the simvastatin effect on HMGCR. A mutated variant of p53 protein present in 50% of colorectal cancer cases reduces KLF2 expression, which leads to the downregulation of p21 protein levels. However, upon simvastatin treatment, the KLF2 level increases and upregulates CDKN1A expression, and downregulates the expression of the mutated variant of TP53, collectively resulting in decreased de novo cholesterol synthesis. (F) KLF9, together with SP5, increases ME1 expression, gene encoding enzyme linking catabolic and anabolic metabolic pathways through NADPH+H+ and leads to an increased de novo synthesis of fatty acids and cholesterol. (G) SP1-dependent expression of β2AR results in an increased phosphorylation of HSL and consequently increased expression of β oxidation-related genes. As a result, the level of triglycerides is reduced, while the levels of free fatty acids and ATP increase.