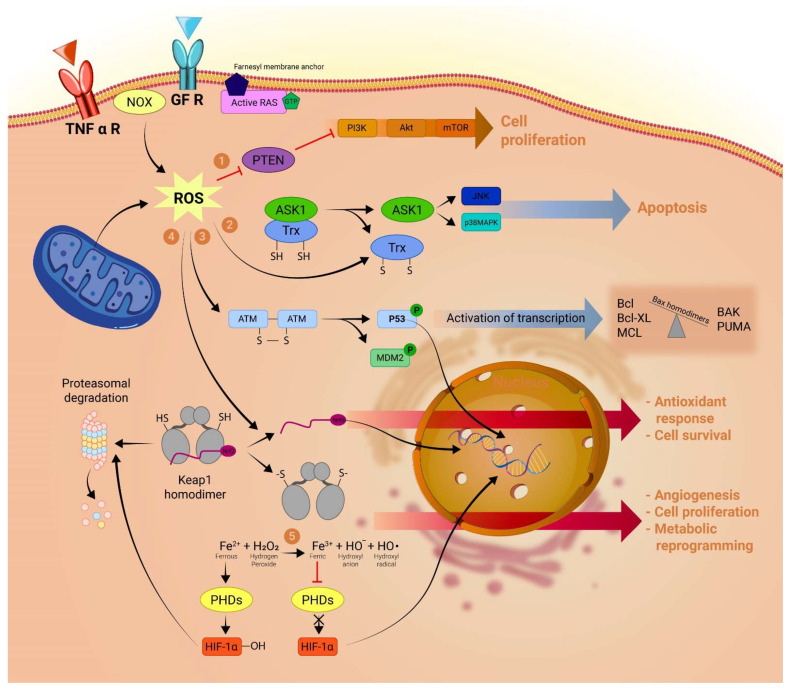
Figure 1.
ROS functions in cancer cells: The production of ROS is elevated in tumor cells as a consequence of increased metabolic rate, gene mutation, extracellular growth factor signaling and relative hypoxia. This increase in the baseline ROS level plays a crucial role in determining cancer cells’ fate: (1) ROS can drive cancer cell proliferation by inactivating PTEN, a tumor suppressor that acts as a brake on the pro-growth PI3K/Akt pathway. (2) Inversely, increased ROS can have a nefarious effect on the cancer cell by activating apoptotic pathways. As such, oxidative stress may lead to the formation of disulfide bonds in the thioredoxin molecule, which results in the activation of the kinase Ask1 and subsequent activation of key apoptotic transcription factors like JNK and p38. (3) A similar process occurs in the case of p53 activation, where an ROS-triggered formation of disulfide bonds at the Cys 2999 residues of ATM homodimer results in activation and subsequent phosphorylation of p53 and its inhibitory molecule MDM2. P53 alongside JNK, p38, and other transcription factors will shift the balance towards apoptosis by expressing proteins, such as Bax homodimers, BAK and PUMA, to the detriment of antiapoptotic proteins such as Bcl-2, Bcl-XL or MCL. (4) ROS can prevent the proteasomal degradation of transcription factor Nrf2 by dissociating the KEAP1-Nrf2 complex and thus allowing Nrf2 to translocate to the nucleus and activate antioxidant pathways that can mitigate the proapoptotic effect of ROS. (5) By oxidizing iron from its ferrous (Fe2+) to its ferric (Fe3+) state, ROS dampens the enzymatic activity of PHDs which would normally hydroxylase the HIF-1α protein causing its proteasomal degradation. The non-hydroxylated form of HIF-1α has a major role in promoting cancer cell survival, angiogenesis and metabolic reprogramming under hypoxic conditions. PTEN, phosphatase and tensin homolog; Ask1, apoptosis signal-regulating kinase 1; ATM, ataxia-telangiectasia mutated; MDM2, mouse double minute 2 homolog; Bax, Bcl-2-associated protein X; Bcl-2, B-cell lymphoma 2; KEAP1, Kelch-like ECH-associated protein 1; PHDs, prolyl hydroxylase domain enzymes; HIF-1α, hypoxia-inducible factor α.