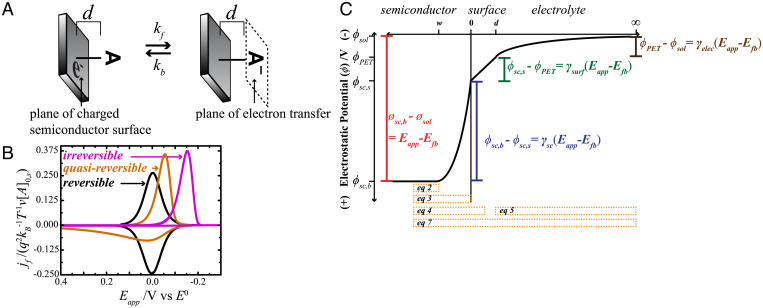
Fig. 1.
A) Simplified depiction of a reducible molecule, A, persistently adsorbed onto a semiconductor surface through a molecular tether of length d. Electron transfer occurs at the plane of electron transfer (PET). (B) Normalized faradaic current vs. applied potential, Eapp, profiles for reversible, quasireversible, and irreversible regimes. (C) A depiction of the electrostatics of the semiconductor–molecule–electrolyte system at an Eapp value that is positive of the flat-band potential, Efb. The total applied potential is partitioned into three regions. The electrostatic potential (ϕ) difference between the semiconductor bulk and surface occurs in the semiconductor space-charge region. The difference in ϕ between the semiconductor surface and the PET occurs in the surface layer. The difference in ϕ between the PET and an infinite distance into the bulk electrolyte occurs in the diffuse layer. The electrostatic potential difference in each region is proportional to the total applied potential by a respective γ-term. The orange boxes denote the Gauss regions that can be defined to generate the respective equations listed in the text.