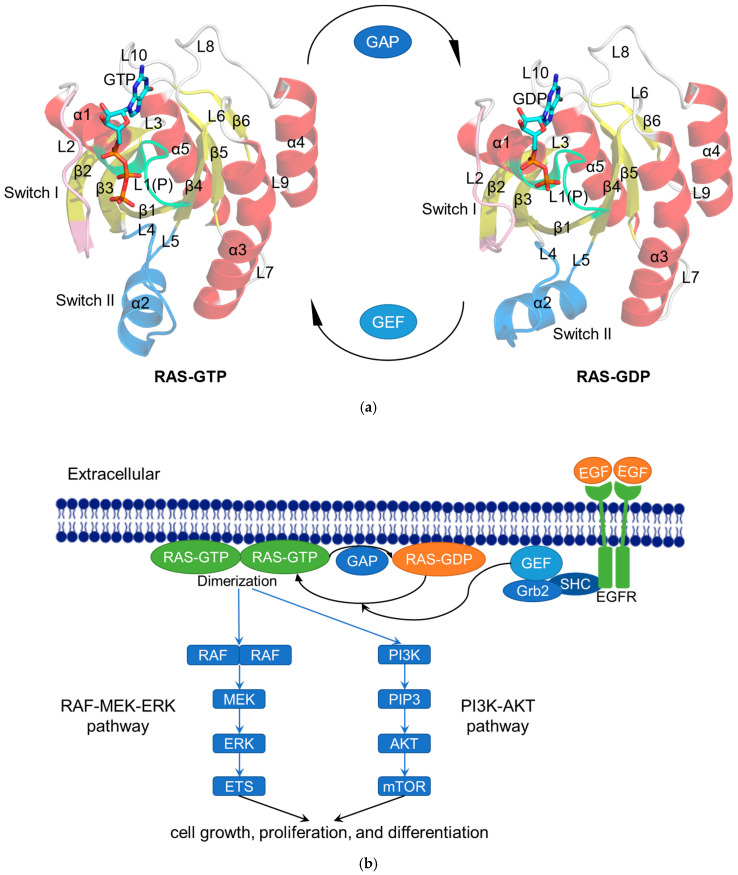
Figure 1.
The RAS functions as a binary switch in normal state. (a) Cartoon representation of the crystal structure of RAS complexes: KRAS4B–GTP (modified from KRAS4B–GppNHp; PDB ID: 3GFT) and KRAS4B–GDP (PDB ID: 4LPK). The helices (α1–α5), strands (β1–β6), and loops (L1–L10) are shown in red, yellow, and gray, respectively. The P-loop (P or L1), Switch I, and Switch II regions are colored lime, pink, and blue, respectively. GTP/GDP are depicted by stick models [23]. (b) Schematic diagram showing the RAS-related signaling pathways. After activation by epidermal growth factor (EGF), the tyrosine kinase receptor EGFR recruits GEF such as SOS to the cell membrane via Src homology 2 domain containing (SHC) and growth-factor-receptor-bound protein 2 (Grb2) to activate RAS [24]. Subsequently, the activated RAS dimerizes and binds to the downstream RAF protein to regulate the MAPK signaling pathway (RAS–RAF–MEK–ERK pathway). The activated ERK is transported to the nucleus and then phosphorylates a number of transcription factors, such as erythroblastosis virus transcription factor (ETS), to ultimately regulate the cell cycle [25]. In another RAS–PI3K–AKT pathway, the activated RAS recruits PI3K to phosphorylate the substrate PIP2 and generate PIP3, whereupon PIP3 causes the sequential phosphorylation of AKT and mTOR to regulate cell proliferation [26].