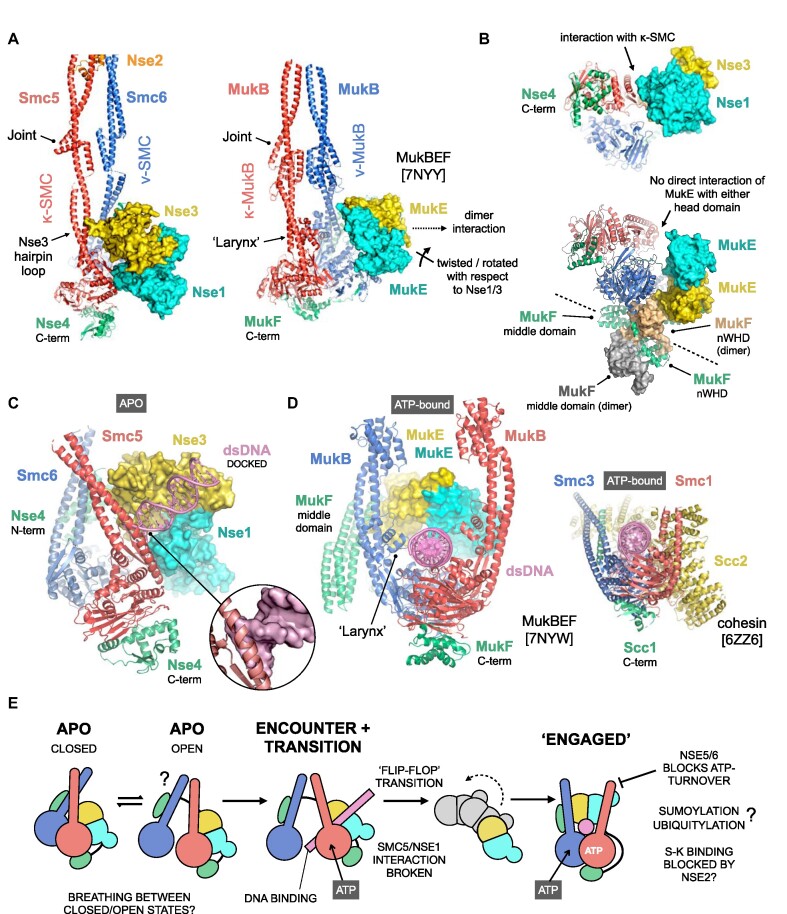
Figure 5.
Exploration of conformational changes likely to accompany binding of dsDNA/ATP. (A) Comparison of the apo-states for Smc5/6 and the related MukBEF complex (PDB: 7NYY). To aid visualisation, only subunits with direct equivalence across both complexes are shown, and for clarity molecular surfaces instead of secondary structure cartoons are shown for the respective KITE proteins. A direct contact between the ‘joint’ features of Smc5 and Smc6 serves to generates a fully ‘closed’ conformation similar to that reported for MukBEF (64) but without a reinforcing secondary ‘larynx’ interface. Furthermore, the KITE homodimer formed by MukE sits, and is held, in a different position when compared to the KITE heterodimer of Nse1/Nse3, largely as a result of its more elaborate interaction with its dimeric kleisin partner MukF. (B) In the apo-state, the MukE heterodimer makes no direct interaction with the head domain of either MukB protein, instead making a series of interactions with the domain-swapped N-terminal winged-helix domain (nWHD) of MukF that anchor it in place. (C) Simple superposition of a DNA duplex, taken from the docking pose reported for the human NSE1/3 heterodimer (65) onto our apo-state structure, indicates that without accompanying conformational changes extension of the trajectory for the bound DNA would generate steric clashes with the arm of Smc5 (inset, DNA now shown in surface representation). (D) DNA/ATP-bound forms of MukBEF (left) and cohesin (inset, right), providing side-by-side comparisons and a visualisation aid of the expected fully ‘engaged’ conformation of SMC-complexes. (E) A speculative model for how the Smc5/6 complex might bind to and engage with dsDNA. We propose that the apo-state can ‘breathe’ between a fully closed conformation and a more open state, which allows / facilitates binding of dsDNA to the positively charged surface / groove created at the interface of Nse1/Nse3, to generate an intermediary ‘encounter’ complex. This, along with concomitant binding of ATP to the head domain of Smc5, serves to break the Smc5/Nse1 interaction allowing a ‘flip-flop’-type transition to the anticipated fully ‘engaged’ state. It is not clear how, or indeed if, ubiquitylation, SUMOylation or other post-translational modification affects either conformation or ATPase activity. It is also not known if the presence of Nse2, acts to block binding or transition of bound dsDNA into the S-K ring (SMC-kleisin) compartment. Binding of the Nse5/6 heterodimer blocks the ability of Smc5/6 to turn over ATP (22,25), but it is not fully known what effect this has on the overall conformation at the head-end of the complex.