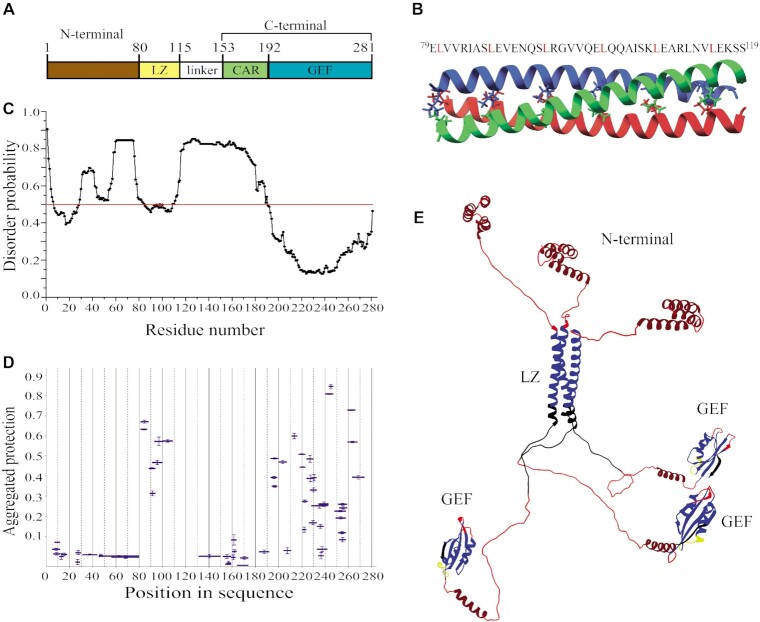
Figure 2.
Structural organization of full-length eEF1Bβ. (A) Domain organization of eEF1Bβ. Abbreviations: LZ – the leucine-zipper motif, CAR – the central acidic region, GEF – the guanine-nucleotide exchange factor domain. (B) Structural model of the LZ-motif built by CCBuilder 2.0. Three α-helices twist around each other to form a bundle with following parameters: radius – 5.6 Å, interface angle – 19º, pitch – 61.8 Å, number of residues per turn – 3.62. The model has the lowest (–425.6 kJ/mol) BUDE score (the interaction energy between the helices). All leucine residues involved onto assembly of the α-helical coiled-coils are shown by colored sticks. The amino acid sequence of the LZ-motif is shown on the top of the figure. (C) Prediction of the disordered regions in eEF1Bβ. All residues whose disorder probability is over 0.5 (red line) are considered as disordered. (D) The aggregated protection plot of eEF1Bβ peptides. The aggregated protection values for peptides (mean ± SD, n = 3 measurements) are plotted versus they position in the protein sequence. (E) The 3D-model of eEF1Bβ colored according to the HDX-MS data. Red color indicates unprotected and unstructured regions (<0.05), dark red – the CAR domain and the N-terminal α-helixes that display no protection, but are predicted to have α-helical organization, yellow – weakly protected dynamic segments (0.05-0.15), blue – highly protected rigidly structured regions (>0.15), black – the regions with missing peptides.