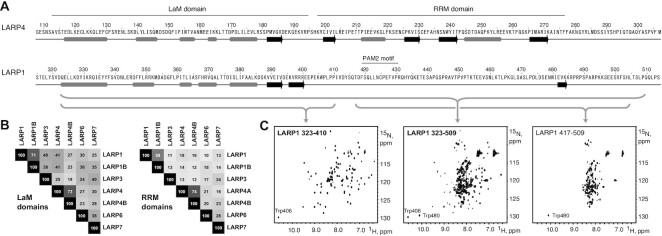
Figure 1.
La-module of LARP1 does not contain an RRM domain. (A) Secondary structure predictions of the LaM and RRM domains of LARP4 and corresponding region of LARP1. Grey bars are alpha helices and black arrows are beta strands. LARP1 contains a PAM2 motif in the region corresponding to the LARP4 RRM but lacks predicted secondary structural elements. See also Supplementary Figure S1. (B) Sequence identity between the LaM and RRM regions of different human LARP proteins. (C) 1H–15N NMR correlation spectra of 15N-labeled LARP1 fragments (numbered according to the 1019-residue long isoform). The spectrum of residues 323–509 (middle spectrum) shows a mix of dispersed signals typical of a folded domain and a central cluster typical of an unfolded protein. Spectra of the separate N- and C-terminal halves confirms that residues 323–410 adopt a folded structure while residues 417–509 are unstructured. See also Supplemetary Figures S2 and S3.