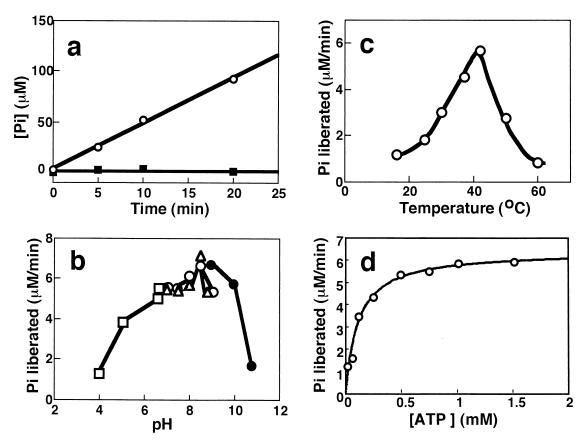
FIG. 6.
Properties of the ATPase activity of AlgSHis6. (a) Linearity of the ATPase reaction at pH 8.0 and 37°C. ○, with 2.5 mM MgCl2; ■, without MgCl2. The final concentration of AlgSHis6 was 0.5 mg/ml. (b) pH dependence at 37°C. Fifty microliters of 100 mM buffers of various pHs containing 10 mM ATP and 5 mM MgCl2 was added to 50 μl of an AlgSHis6 (1.2 mg/ml) solution dialyzed against 5 mM Tris-HCl (pH 8.0)–0.1 mM EDTA–20% glycerol. □, sodium acetate; ○, Tris-HCl; ▵, sodium HEPES; ●, sodium CAPSO. (c) Temperature dependence at pH 8.0 (HEPES). The final concentration of AlgSHis6 was 0.5 mg/ml. (d) Dependence on the ATP concentration at pH 8.0 and 37°C. Fifty microliters of various amounts of an ATP and MgCl2 (molar ratio, 2:1) mixture solution was added to 50 μl of an AlgSHis6 solution (0.9 mg/ml) including 0.05 mM EDTA and 20% glycerol. The solid line is the theoretical curve for Km = 0.11 mM and Vmax = 6.42 μM/min.