Abstract
Objective
To investigate the effect of artificial skin on the expression of miR‐155 and miR‐506‐3p in patients with second‐degree burns.
Methods
The study subjects included 50 patients with second‐degree burns treated from July 2019 to July 2021. The control group received routine nursing, while the research group received both routine and artificial skin intervention simultaneously. The changes in wound tissue fibrosis and prognosis were observed. The expression levels of miR‐155 and miR‐506‐3p and their downstream regulatory factors were detected and correlated with the rehabilitation of patients after artificial skin treatment.
Results
After treating second‐degree burns with artificial skin membranes, the patient's wound tissue fibrosis and inflammation level improved. At the same time, the expression levels of miR‐155 and miR‐506‐3p in related tests were higher than those in patients with available treatment.
Conclusion
The effect of artificial skin membrane on the wound healing of second‐degree burn patients may be realized by influencing the expression levels of miR‐155 and miR‐506‐3p and their related signaling pathways.
Keywords: artificial skin, burn, microRNA155, microRNA‐506‐3p
Artificial skin membrane can effectively stimulate the expressionof miR‐155and miR‐506‐3p in wound skin cells, reduce the level of fibrosis in second‐degree burn wounds through PI3K‐AKT pathway, and promote wound repair and healing.
1. INTRODUCTION
The insufficient treatment of second‐degree burn wounds is a problem that has plagued patients and their families in the burn department. Most second‐degree burns are caused by fire, high temperature, and electric shock. Patients usually heal slowly, and even after the burned skin heals, it is transformed into fibrous scar tissue, 1 , 2 accompanied by excessive proliferation of fibroblasts and apoptosis of keratinocytes. 3 , 4 Unfortunately, scars, depigmentation in the affected area, and deformation in the process of skin prognosis are quite common. The inflammatory reaction caused by severe burns can cause further damage to the burned skin tissue and significantly impact the patient's daily life. At present, the clinical effect of routine drug treatment is poor, and patients' satisfaction is low. 5 , 6 Therefore, it is of great significance to study the mechanism of artificial skin membranes and their related gene therapy targets.
MicroRNAs are a biomarker widely used for disease prevention, diagnosis, and testing prognosis. It can affect autoimmune development and immune cells by regulating gene expression after mRNA transcription and most cellular processes, including cell proliferation, differentiation, development, metabolism, apoptosis, signal transduction, and immune response. 7 , 8 The abnormal expression of miRNA is related to the pathogenesis of various diseases. 9 , 10 , 11 miR‐506‐3p is a short‐stranded noncoding RNA located in the third sub‐band of the seventh band in the second region of the long arm of the X chromosome. It often plays various roles in different diseases. Some studies have pointed out that miR‐506‐3p, as a tumor suppressor gene, is negatively correlated with lymphatic tumor metastasis and distant metastasis in the occurrence and development of some tumor diseases. For example, in colorectal tumors, miR‐506‐3p takes Lamc1 as the inhibitory target to regulate the proliferation and invasion of tumor cells; however, in other diseases, such as melanoma, miR‐506‐3p is expressed as a cancer‐promoting gene. 12 , 13 , 14 , 15 In addition, some studies point out that the change in miR‐506‐3p expression level is characterized by the level of autophagy of heat‐damaged fibroblasts. 16 , 17 , 18 As another short‐chain noncoding RNA, miR‐155 widely exists and acts on various skin diseases. 19 , 20 , 21 , 22 Some studies have reported that miR‐155 can target and activate CD8+ cells, inhibit cytokine signal transduction and melanin production through interferon, and act on related skin diseases. 23 , 24
At present, there are few reports on the therapeutic effect and mechanism of artificial skin film in supporting second‐degree burn patients. This study selected 50 second‐degree burn patients in our hospital as the research subject to explore the effect of artificial skin film on the expression of miR‐506‐3p and miR‐155 in burn patients to provide a reference for subsequent clinical treatment. The specific reports are as follows.
2. MATERIALS AND METHODS
2.1. Clinical information
Fifty patients with second‐degree burns who met the experimental requirements admitted to at the Department of Emergency of the Xuancheng People's Hospital from July 2019 to July 2021 were randomly divided into research and control groups. The second‐degree burn wounds in the research group were treated with haifukang artificial skin membrane nursing. Meanwhile, the control group was wrapped with Vaseline gauze. Three independent pathologists verified the diagnosis of the patients. All the enrolled patients must be hospitalized within 2 h of injury. They did not receive any treatment within 1 year before the operation and had no history of tumors; Patients with any other type of substantial organ dysfunction were excluded. This experiment has been authorized by the Ethics Committee of Xuancheng People's Hospital. The patient's guardians signed the informed consent.
2.2. Cell lines
Under sterile conditions, 100 samples of patients within 24 h after burn injury were obtained. The specimens were placed in cryopreservation tubes and quickly frozen using liquid nitrogen. Afterward, the tubes were maintained in a refrigerator at −80 for long‐term storage. The human normal skin cell line (Hsas4) cell heat injury model was established. This cell line (Hsas4) was sourced from Shanghai Enzyme‐linked Biotechnology Co., LTD. All cell lines were cultivated in Dulbecco's Modified Eagle Medium (DMEM) with additional 10% fetal bovine serum (FBS; Hyclone) and 1% penicillin/streptomycin (Beyotime). The cultures were maintained at 37°C in an incubator filled with 5% CO2. After normal passage, logarithmic cells of more than 3 generations were selected for subsequent experiments.
2.3. Prognostic analysis
Both groups were given routine nursing after admission. The patient underwent simple debridement, and the wound was repeatedly washed with sterile normal saline and dried. After that, 1% silver sulfadiazine cream was evenly applied. Both groups used the corresponding covering, semi‐exposed, and treated with far infrared. In both groups, the coverings were changed on the 3rd day after surgery, and once every 2–3 days until the wound healed. Wound healing quality was evaluated after 3 months of treatment. Patients in both groups were followed up for half a year to observe whether there was pigmentation and scar hyperplasia.
2.4. Immunohistochemical test
The specimens' tissue slices were incubated with 0.3% endogenous peroxidase blocking solution for 20 min after dewaxing and hydration, then they were incubated with 3% hydrogen peroxide solution at room temperature for 10 min. The slices were washed with PBS three times (3 min/time). Antigen retrieval was performed using citrate buffer (pH 6.0) at 121°C for 2 min. The cells were incubated with primary monoclonal antibodies (1:100 anti‐Col1A1, and 1:100 anti‐α‐SMA) at 4°C overnight after blocking with 5% BSA for 2 h at room temperature. The cells were then sequentially incubated with rabbit anti‐mouse and goat anti‐rabbit non‐biotinylated regents (Zhongshanjinqiao) according to the manufacturer s instructions, and they were observed under a microscope and analyzed with a photo and image auto analysis system (Image‐Pro Plus).
2.5. qRT‐PCR
The tissues and cells were lysed using TRIzol reagent (TaKaRa), and the extracted RNA samples were reverse transcribed into cDNA using PrimeScript RT Master Mix (TaKaRa). PCR amplification was performed on an ABI 7500 thermocycler (Applied Biosystems) using SYBR Green (Thermo Fisher scientific). The relative expression levels of mRNAs and miR were calculated and quantified using the 2 ΔΔCT methods after normalization with β‐actin and U6 levels, respectively (The primer sequences for target gene and endogenous references are shown in Table 1). 13 The experimental operation was repeated three times independently.
TABLE 1.
The primer sequences included in the study
| Name | Primer sequences (5′–3′) |
|---|---|
| miR‐155 | |
| Forward | GCGTATAGCTTTCGCGCATATA |
| Reverse | TAGCTAGCGTAGCTCAGCTTTG |
| miR‐506‐3p | |
| Forward | TAAGGC‐ACCCTTCTGAGTAGA |
| Reverse | GCGAGC‐ACAGAATTAATACGAC |
| α‐SMA | |
| Forward | TGGCCACTGCTGCTTCCTCTTCTT |
| Reverse | GGGGCCAGCTTCGTCATACTCCT |
| Col1A1 | |
| Forward | GGAGAGAGCATGACCGATGG |
| Reverse | GGGACTTCTTGAGGTTGCCA |
| β‐actin | |
| Forward | CCTGTACGCCAA‐CACAGTGC |
| Reverse | ATACTCCTGCTTG‐CTGATCC |
| U6 | |
| Forward | GT‐GCTCGCTTCGGCAGCACAT |
| Reverse | TACCTTGCGAAGTGCTTAAAC |
2.6. Western blotting
Total protein was extracted from the cells after they were transfected for 48 h. The transfected cells were lysed with radioimmunoprecipitation assay lysis buffer (PBS containing 1% NP40, 0.1% SDS, 5 mM EDTA, 0.5% sodium deoxycholate, 1 mM sodium orthovanadate, and protease inhibitors) on ice for 30 min with shaking at 12,000 rpm/min. Total proteins were isolated, and the concentration was determined using a BCA Kit. Proteins (40 μg/lane) were separated using 10% SDS‐PAGE electrophoresis. Then, the gel was electrotransferred to a PVDF membrane. The PVDF membrane was rinsed with TBS for 10–15 min, placed in TBS/T blocking buffer containing 5% (w/v) skimmed milk powder, and shaken at room temperature for 1 h. The membrane was incubated at 4°C overnight after adding primary antibodies. Then, the membrane was washed with TBST three times (5 min each time). The membrane was incubated at 37°C for 1 h with IgG‐HRP secondary antibody (1:10,000). The membrane was developed with ECL (Perkin‐Elmer Inc.) for 5 min. The protein bands were quantified as a ratio to β‐actin using ImageQuant LAS4000 (GE Healthcare).
2.7. Cell transfection
The mimics and inhibitors of two target miRNAs were synthesized by GenePharma Biotechnology. And then, the target miRs mimics, inhibitors, or negative control miRNA (NC) was transfected into Hsas4 cell using Lipofectamine 2000 transfection reagent according to the manufacturer s instructions. After 48 h transfection, the non‐treated or treated cells were collected for further experiments.
2.8. Cell counting kit‐8 (CCK‐8) assay
With 1 × 103 cells/well density, the cell suspension was inoculated into a culture plate with 96‐wells and then maintained at 37°C in an incubator with 5% CO2. After transfection, the cells were cultured for 0, 24, 48, 72 h. Afterward, 10 μl CCK‐8 reagent (Beyotime) was pipetted into the wells, then the cells were cultivated for another hour. A microplate reader (Thermo Fisher Scientific) was utilized to measure the absorbance of each well at a 450 nm wavelength. Finally, the curve for cell growth was constructed.
2.9. Dual‐luciferase reporter assay
Possible binding sites were screened with the help of the bioinformatics tools Target Scan(http://www.targetscan.org/vert_72/). It was found that the 5′ terminus of miR‐155 and miR‐506‐3p specifically bind to 3′‐UTR of PIK3CA, which is the gene encoding the catalytic subunit P110 of PI3K in the PI3K‐Akt pathway. The binding interactions between PIK3CA and miR‐155 or miR‐506‐3p were confirmed by conducting dual‐luciferase reporter assays. PIK3CA wild‐type (WT) and mutant (MUT); miR‐mimic and NC control were synthesized by Suzhou Jima Gene Co., LTD (The primer sequences for PIK3CA‐WT and PIK3CA‐MUT references are shown in Table 2). The Hsas4 cell was transfected with the different vectors with the aid of a LipofectamineTM 2000 Transfection Reagent (Beijing Solaibao Technology Co., LTD). After the 48‐h transfection, the luciferase activities of firefly and renilla were measured by means of a dual‐luciferase reporter assay kit (Beijing Solaibao Technology Co., LTD).
TABLE 2.
Target gene PIK3CA 3‐UTR primer
| Name | Primer sequences (5′–3′) | Fragment length/bp | Tm/°C |
|---|---|---|---|
| PIK3CA‐WT | 330 | 62 | |
| Forward | GGACTAGTGAAAATGAAAGCTCA | ||
| Reverse | CGAGCTCCTTCTCCATCATTTCTAT | ||
| PIK3CA‐MUT | 235 | 63 | |
| Forward | GGACTAGTGAAAATGAAAGCTCACTC | ||
| Reverse | CGAGCTCTATAAGATAACATGAAATTGCGCATT |
2.10. Statistical analysis
The relevant data obtained in this study were collected by professional investigators and imported into SPSS 22.0 statistical analysis. The measurement data are expressed as the mean ± standard deviation. The intergroup comparison was performed with one‐way ANOVA. The counting data are expressed as percentages (%), and the matching data were compared by t‐test, χ2 analysis, and comparison between groups. The difference was statistically significant (p < 0.05).
3. RESULTS
3.1. Comparison of prognosis between the two groups
After comparing the prognosis of wound between the two groups, it was found that the wound healing time in the research group (16.3 ± 2.4 days) was significantly shorter than that in the control group (19.5 ± 2.8 days; p < 0.05); there were 5 cases of wound pigmentation and 4 cases of scar hyperplasia in the research group (p < 0.05); in the control group, there were 10 cases of pigmentation and 13 cases of scar hyperplasia, indicating that the effect of artificial skin film on the recovery of patients after burn injury is better (Figure 1A; Table 3).
FIGURE 1.
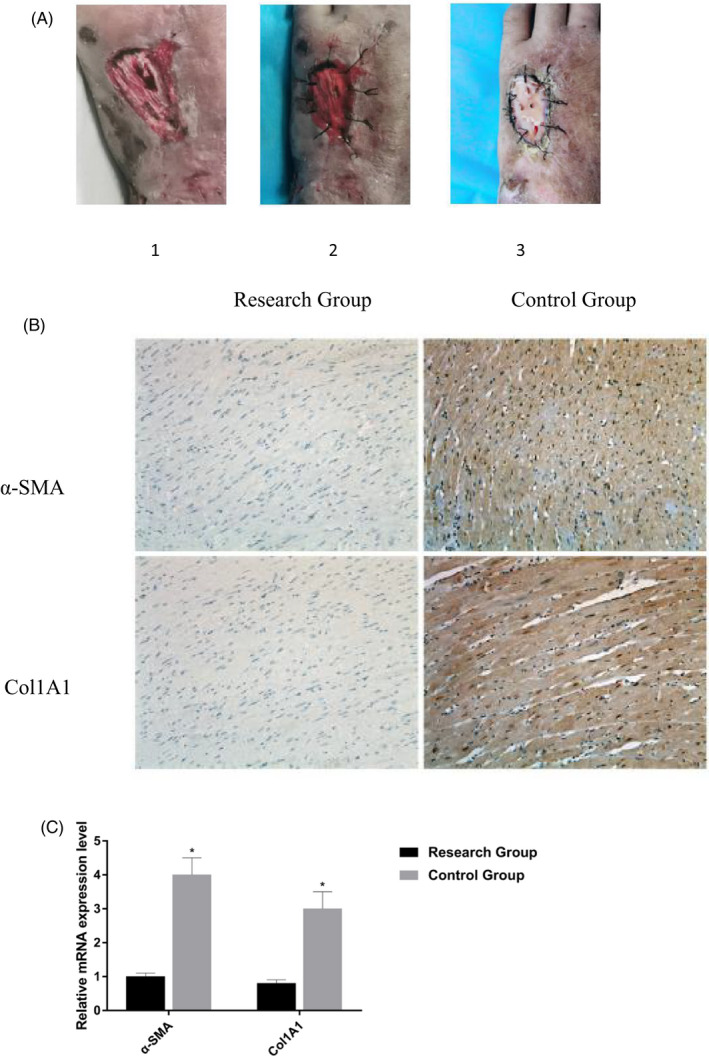
Comparison of wound and the changes of Immunohistochemistry. (A) After the same debridement application, the study group was given haifukang artificial skin film combined. 1. The wound surface of the patient's preoperative second‐degree burn. 2. Haifukang artificial skin film was used to directly cover the wound. 3. The second‐degree burn wound was well vascularized 4 weeks after artificial skin membrane transplantation. (B) Immunohistochemistry results showed that the protein expression levels of Col1A1 and α‐SMA in the control group were higher than those in the research group. (C) The mRNA expression levels of Col1A1 and α‐SMA decrease in the research group.
TABLE 3.
Prognosis of two groups of patients
| Groups | Examples(n) | Healing time(d) | Pigmentation | Scar hyperplasia |
|---|---|---|---|---|
| Research Group | 25 | 16.3 ± 2.4** | 5* | 4** |
| Control Group | 25 | 19.5 ± 2.8 | 10 | 13 |
| t and χ2 | 5.812 | 3.281 | 5.162 |
Comparison with the control group, p < 0.05.
Comparison with the control group, p < 0.01.
3.2. The changes in Immunohistochemistry
Col1A1 and α‐SMA are landmark proteins reflecting the degree of tissue fibrosis. Immunohistochemistry results showed that the protein expression levels of Col1A1 and α‐SMA in the control group were higher than those in the research group. qRT‐PCR results showed that the mRNA expression levels of Col1A1 and α‐SMA decreased in the research group. The difference was statistically significant (p < 0.05; Figure 1B,C).
3.3. Expression of miR‐506‐3p and miR‐155 in cells by qRT‐PCR
The expression levels of miR‐506‐3p and miR‐155 in burn tissues and surrounding normal tissues of 50 patients were detected by qRT‐PCR. The results showed that compared with normal tissues, the expression of miR‐506‐3p and miR‐155 in burn tissues decreased (p < 0.05; Figure 2A,B). The expression levels of miR‐506‐3p and miR‐155 in cured tissues of 2 different groups were detected by qRT‐PCR. The results showed that compared with the research group, the expression of miR‐506‐3p and miR‐155 in the control group decreased (p < 0.05). The difference was statistically significant (p < 0.05; Figure 2C,D).
FIGURE 2.
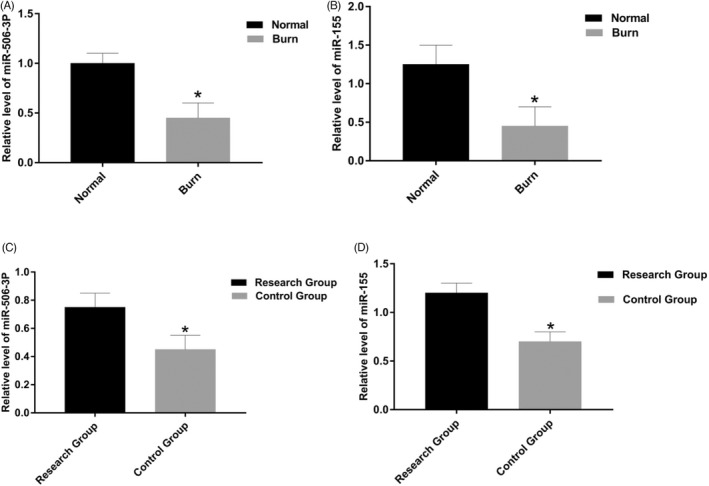
Expression of miR‐506‐3p and miR‐155 by qRT‐PCR. (A) The expression of miR‐506‐3p in burn tissues decreased (p < 0.05). (B) The expression of miR‐155 in burn tissues decreased (p < 0.05). (C) In cured tissues, the expression of miR‐506‐3p in control group is lower than research group (p < 0.05). (D) In cured tissues, the expression of miR‐155 in control group is lower than research group (p < 0.05).
3.4. Western blot analysis
Western blot analysis detected the expression of Col1A1 and α‐SMA in each group after artificial skin membrane treatment. The results showed that the expression of Col1A1 and α‐SMA in patient with second‐degree burns tissues after artificial skin membrane treatment was significantly lower than that before treatment (p < 0.05; Figure 3A,B). After artificial skin membrane treatment, TNF‐α, IL‐6, and IL‐8 were found in the serum by comparing the inflammatory factors. The levels of TNF‐α, IL‐6, and IL‐8 decreased significantly, and the difference was statistically significant (p < 0.05; Figure 3C).
FIGURE 3.
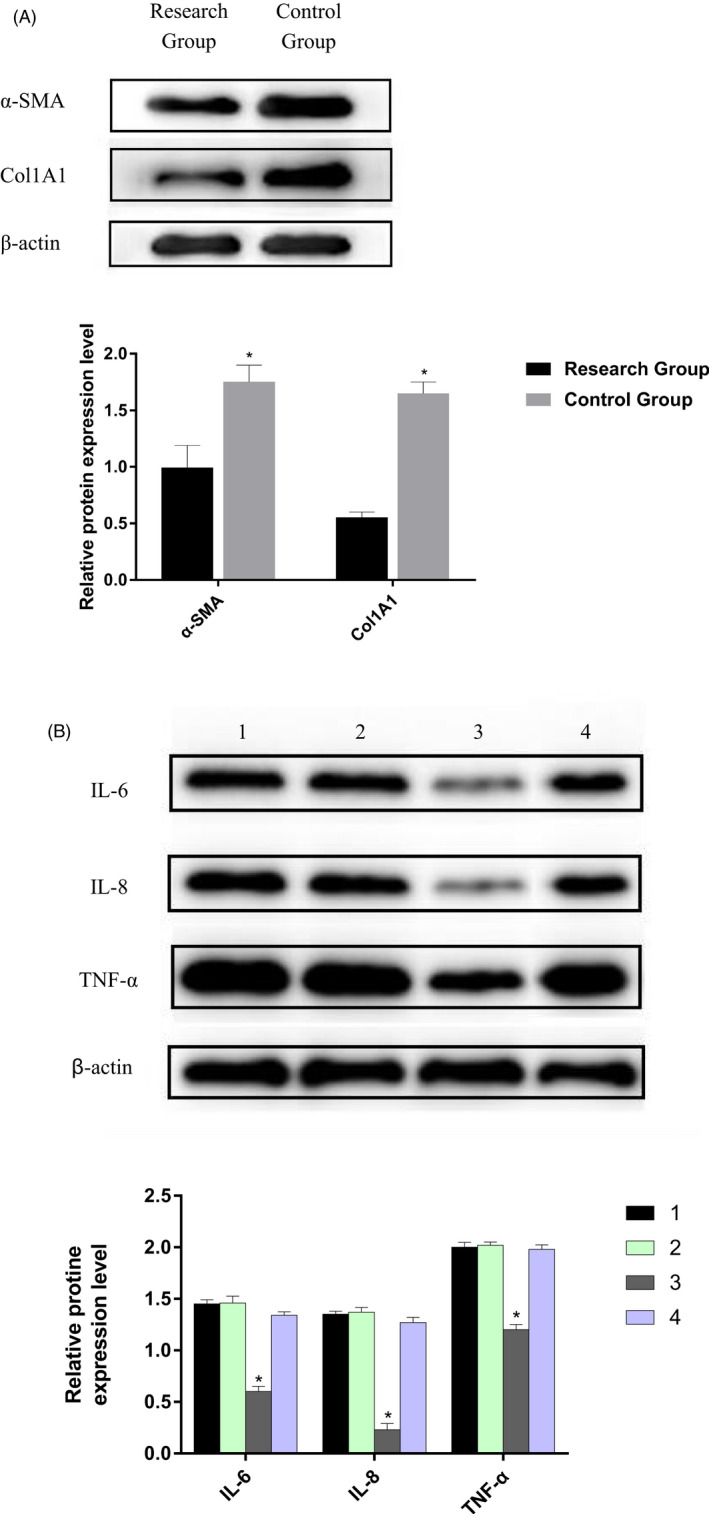
Western blot analysis detected the expression of fibrosis marker proteins and inflammatory factors in each group. (A) The expression of Col1A1 and α‐SMA in patient second‐degree burns tissues after artificial skin membrane treatment was significantly lower than that before treatment (p < 0.05). (B) The levels of TNF‐α, IL‐6 and IL‐8 decreased significantly in research group, and the difference was statistically significant (p < 0.05). 1. The pre‐treatment research group. 2. The pre‐treatment control group. 3. The research group after treatment. 4. The control group after treatment.
3.5. miR‐506‐3p and miR‐155 via targeting PIK3 CA of PI3K‐AKt pathway
TargetScan was used to identify the potential targets of miR‐506‐3p and miR‐155. An important gene in PI3K‐AKt pathway, PIK3CA, was identified as one of the potential targets of miR‐506‐3p and miR‐155 (Figure 4A). To examine miR‐506‐3p–PIK3CA and miR‐155–PIK3CA interactions, PIK3CA complementary sites, with or without mutations, were cloned into the 3′‐UTR of the firefly luciferase gene and co‐transfected with miR‐506‐3p and miR‐155 mimics or a negative control in Hsas4 cell. Recombinant plasmids P miR‐Report‐PIK3CA‐WT and P miR‐Report‐PIK3CA‐MUt (Figure 4B). Results depicted that miR‐506‐3p mimic and miR‐155 mimic reduced PIK3CA‐WT's luciferase activity, yet it had very little influence on PIK3CA‐MUT's luciferase activity (Figure 4C). After transfection of miR‐506‐3p and miR‐155 mimics and inhibitors into Hsas4 cells, Western blot analysis showed that miR‐506‐3p and miR‐155 had targeted inhibitory effect on PIK3CA. The difference was statistically significant (p < 0.05; Figure 4D,E).
FIGURE 4.
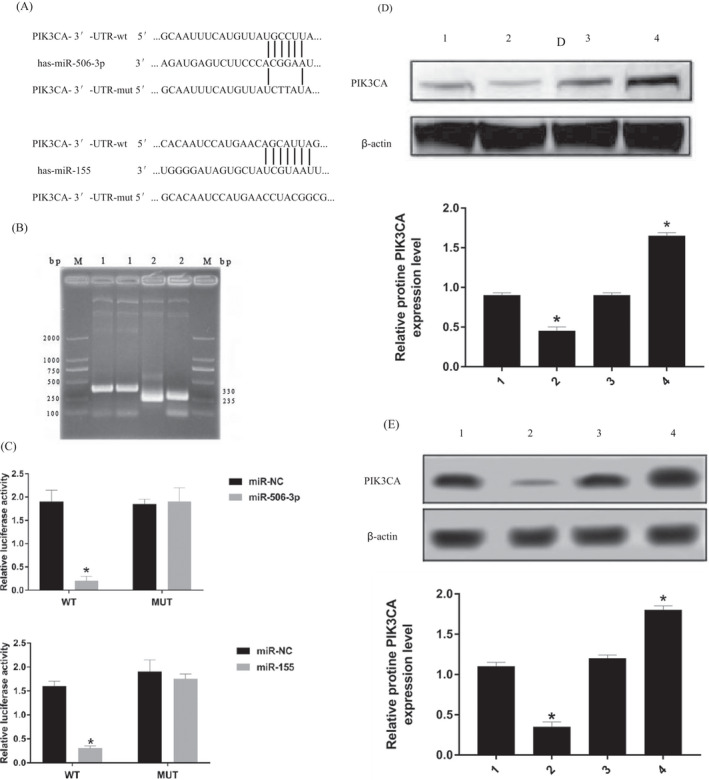
miR‐506‐3p and miR‐155 can target regulation of the PI3K‐Akt pathway. (A) PIK3CA, was identified as one of the potential targets of miR‐506‐3p and miR‐155. (B) Recombinant plasmids P miR‐Report‐PIK3CA‐WT and P miR‐Report‐PIK3CA‐MUt. The length of p miR‐Report was 6470 bp, and PCR showed two fragments of the recombinant plasmid: PIK3CA‐WT 330 bp/PIK3CA‐MUT 235 bp. Agarose gel electrophoresis showed that the exogenous gene was successfully inserted. (C) miR‐506‐3p mimic and miR‐155 mimic reduced PIK3CA‐WT's luciferase activity, yet it had very little influence on PIK3CA‐MUT's luciferase activity. (D) 1. mimic‐N.C; 2. miR‐506‐3p mimic; 3. inhibitors‐N.C; 4. miR‐506‐3p inhibitor. After transfection of miR‐506‐3P mimic into Hsas4 cells, the expression of PIK3CA protein was lower than that of mimic‐N.C (p < 0.05), PIK3CA protein expression in miR‐506‐3p inhibitor group was higher than that in inhibitors‐NC (p < 0.05). (E) 1. mimic‐N.C; 2. miR‐155 mimic; 3. inhibitors‐N.C; 4. miR‐155 inhibitor. After transfection of miR‐155 mimic into Hsas4 cells, the expression of PIK3CA protein was lower than that of mimic‐N.C (p < 0.05), PIK3CA protein expression in miR‐155 inhibitor group was higher than that in inhibitors‐NC (p < 0.05).
3.6. miR‐506‐3p and miR‐155 inhibit Hsas4 cell proliferation through PI3K/AKT signaling pathway
miR‐506‐3p and miR‐155 mimics at a concentration of 10 nm were transfected in vitro to detect the proliferation of Hsas4 cell. The results showed that after transfection with miR‐506‐3p and miR‐155 mimics, the proliferation of Hsas4 cell decreased significantly. However, on the basis of transfecting the miR‐506‐3p and miR‐155 mimics, the proliferation level of Hsas4 cells relatively increased with the addition of IGF‐1(Beyotime, Shanghai, China), a PI3K/AKT signaling pathway activator. The results showed that the overexpression of miR‐506‐3p and miR‐155 could inhibit the proliferation of Hsas4 cell. And PI3K/AKT signaling pathway activator IGF‐1 reversed the proliferation inhibition of miR‐506‐3p and miR‐155 on Hase cells. The difference was statistically significant (p < 0.05; Figure 5).
FIGURE 5.
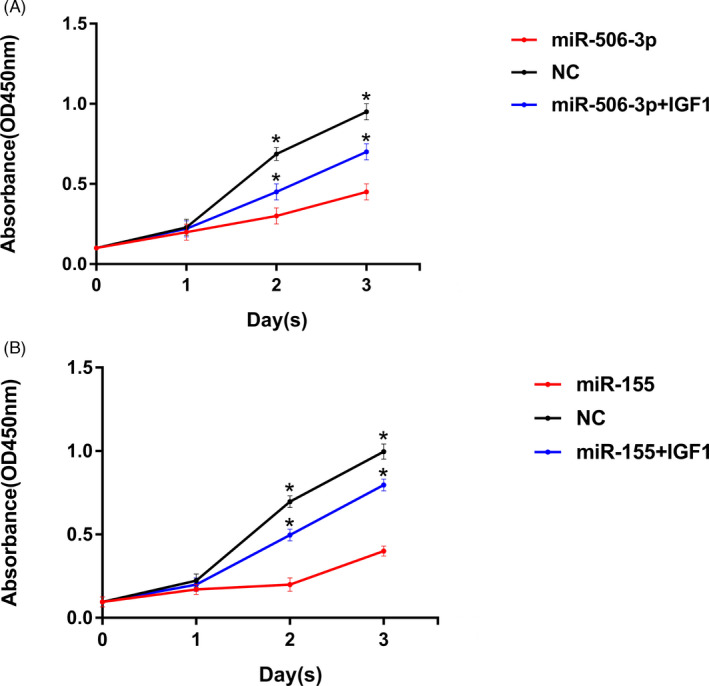
(A) After overexpression of miR‐155, the proliferation level of Hsas4 cells in the research group was lower than that in the control group at the same time point. However, after the addition of IGF‐1 (An Activator of PI3K/AKT Signaling pathway), compared with the research group, the proliferation level of Hsas4 cells in the IGF‐1 group increased. (B) After overexpression of miR‐506‐3P, the proliferation level of Hsas4 cells in the research group was lower than that in the control group at the same time point. However, after the addition of IGF‐1 (An Activator of PI3K/AKT Signaling pathway), compared with the research group, the proliferation level of Hsas4 cells in the IGF‐1 group increased.
4. DISCUSSION
The human body will not reject Haifukang artificial skin film. It has the characteristics of easy preservation, convenient material selection, and a simple method. The Haifukang PI membrane contains chitosan and other extracted components and has good histocompatibility. During treatment, epidermal growth factor 25 , 26 can promote the growth of epidermal cells, block bacteria, and prevent infection. It is widely used in the treatment of burn patients, but the specific mechanism and treatment focus are unclear.
Scar is the most common posttraumatic manifestation in dermatology and plastic surgery. It is a significant problem in posttraumatic patients. Finding the mechanism and treatment target of scar formation is an important objective of clinical treatment. Second‐degree burns often lead to injury to the dermal reticular layer and the necrotic tissue of burn wounds persists and promotes infection breeding in follow‐up treatment. In early treatment, surgery is often used to remove necrotic tissue, so there are problems such as secondary injury or unclean removal, resulting in secondary and persistent infection and affecting wound healing. Scar formation is caused by abnormal proliferation of fibroblasts and excessive deposition of extracellular matrix proteins. Fibroblasts are the primary effector cells of scar formation. The study of the proliferation level of fibroblasts is of great significance for scar prevention and treatment. Many studies show that miRNAs regulate the biological effects of fibroblast proliferation, differentiation, and apoptosis and have an abnormal expression in scar formation. 27 For example, miR‐181a regulates apoptosis and expansion of human keloid fibroblasts through phlpp. 25 miR‐181b‐5p promotes proliferation and inhibits apoptosis of hypertrophic scar fibroblasts by regulating the MEK/ERK/p21 pathway. 28
Re‐epithelialization is one of the fundamental characteristics of wound healing and depends on two basic functions of fibroblast: proliferation and migration. After examining the proliferation of fibroblasts and the recovery of patients, it was found that miRNA could significantly reduce the size of wounds in vivo and accelerate wound healing by increasing the re‐epithelialization of wounds, myofibroblasts, and collagen deposition. A few days before treatment, IL‐6 and IL‐8 showed an apparent upward trend, closely related to fibrotic diseases. IL‐6 and IL‐8, as the critical factors of acute inflammatory reactions, can be significantly increased in various types of infectious diseases. Especially in severe cases, it is urgent to reduce inflammatory factors and reduce the side effects of inflammatory reactions in patients.
This study found that the expression of miR‐506‐3p and miR‐155 was significantly downregulated in skin fibroblasts of burned skin tissue, suggesting that miR‐506‐3p and miR‐155 are significantly associated with scar formation and can be used as potential targets for the diagnosis and treatment of scar and fibrotic diseases. After haifukang artificial skin membrane treatment, miR‐506‐3p and miR‐155 recovered significantly (p < 0.05), which was significantly higher than that before treatment. After transfection of miR‐506‐3p and miR‐155 simulants, the viability of fibroblasts was significantly reduced in a dose‐dependent manner, indicating that miR‐506‐3p and miR‐155 inhibit the proliferation of fibroblasts and slow down the formation of scars. By comparing the inflammatory factors, the levels of TNF‐α, IL‐6 and IL‐8 decreased significantly after treatment with artificial skin membrane (p < 0.05). The results showed that haifukang artificial skin membrane treatment could significantly improve the rehabilitation level of patients, and the levels of miR‐506‐3p and miR‐155 increased, which was helpful for cells to inhibit the expression of inflammatory factors such as TNF‐α, IL‐6 and IL‐8 promotes body reparability.
Also, this study explored the possible co‐regulated signaling pathways downstream of these two miRNAs. We found that the 5' terminus of miR‐506‐3p and miR‐155 specifically bind to 3′‐UTr of PIK3CA, which is the gene encoding the catalytic Subunit P110 of PI3K in the PI3K‐Akt pathway. And through a series of experimental verification, miR‐506‐3p and miR‐155 may affect the occurrence and development of wound fibrosis through targeting the PI3K‐Akt pathway. However, recent studies also have found that miR‐506‐3p can regulate the behavior of burn tissue fibroblasts by targeting beclin‐1. 29 After transcription, miR‐506‐3p inhibited beclin‐1 expression. Beclin‐1 is crucial autophagy promoting protein. Downregulation of beclin‐1 can significantly inhibit fibroblast activity and collagen synthesis. miR‐506‐3p targets and regulates autophagy and proliferation of skin fibroblasts after burn injury, thereby inhibiting the formation of hypertrophic scars. miR‐155 increased significantly after epidermal injury. The mechanism may be that inflammatory cytokines regulate the inflammatory response, cause the activation of melanocytes and keratinocytes, and promote the expression of miR‐155. Future experiments can also explore the targets of beclin‐1 and melanocytes and further study the mechanisms and targets of miRNA and autophagy to help better understand its anti‐scar effect.
5. CONCLUSION
Haifukang artificial skin membrane therapy can effectively stimulate the expression of miR‐155 and miR‐506‐3p, and promote wound recovery of second‐degree burn patients through PI3K‐Akt signaling pathway.
AUTHOR CONTRIBUTIONS
Fei Li and Runhe Qin performed the experiments and data analysis. Fei Li and Runhe Qin conceived and designed the study and acquired the data. Runhe Qin performed data analysis and interpretation. All authors have read and approved the manuscript.
Funding information
Funding information is not available.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
CONSENT TO PARTICIPATE
All patients signed written informed consent.
CONSENT FOR PUBLICATION
Consent for publication was obtained from the participants.
CODE AVAILABILITY
Not available.
ACKNOWLEDGMENTS
None.
Li F, Wan DW, Hu J, Qin R. Effect of artificial skin membrane on the expression of miR‐155 and miR‐506‐3p in patients with second‐degree burns. J Clin Lab Anal. 2022;36:e24564. doi: 10.1002/jcla.24564
Fei Li and Runhe Qin contributed equally to this work.
DATA AVAILABILITY STATEMENT
The datasets that have been used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Song KX, Liu S, Zhang MZ, et al. Hyperbaric oxygen therapy improves the effect of keloid surgery and radiotherapy by reducing the recurrence rate. J Zhejiang Univ Sci B. 2018;19(11):853‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tredget EE, Shupp JW, Schneider JC. Scar management following burn injury. J Burn Care Res. 2017;38(3):146‐147. doi: 10.1097/BCR.0000000000000548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang X, Wang R, Li F, Wu Y, Liu Y, Zhang W. Relationship between miR‐21 and miR‐182 levels in peripheral blood and gastric cancer tissue. Oncol Lett. 2017;14:1427‐1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang J, Li Y, Bai X, Li Y, Shi J, Hu D. Recent advances in hypertrophic scar. Histol Histopathol. 2018;33(1):27‐39. doi: 10.14670/HH-11-908 [DOI] [PubMed] [Google Scholar]
- 5. Liang PF, Zhang PH, Zhang MH, et al. Repair methods and clinical effects of full‐thickness burn wounds deep to tendon or even bone in fingers. Zhonghua shao shang za zhi = Zhonghua shaoshang zazhi = Chinese Journal of Burns. 2021;37:1‐8. [DOI] [PubMed] [Google Scholar]
- 6. Ault P, Plaza A, Paratz J. Scar massage for hypertrophic burns scarring‐a systematic review. Burns. 2018;44(1):24‐38. doi: 10.1016/j.burns.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 7. Shang Z, Li H. Altered expression of four miRNA (miR‐1238‐3p, miR‐202‐3p, miR‐630 and miR‐766‐3p) and their potential targets in peripheral blood from vitiligo patients. J Dermatol. 2017;44:1138‐1144. [DOI] [PubMed] [Google Scholar]
- 8. Yang L, Yang S, Lei J, et al. Role of chemokines and the corresponding receptors in vitiligo: a pilot study. J Dermatol. 2017;45:31‐38. [DOI] [PubMed] [Google Scholar]
- 9. Zheng YZ, Chen CF, Jia LY, Yu TG, Sun J, Wang XY. Correlation between microRNA‐143 in peripheral blood mononuclear cells and disease severity in patients with psoriasis vulgaris. Oncotarget. 2017;8:51288‐51295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dang X, Qu X, Wang W, et al. Bioinformatic analysis of microRNA and mRNA Regulation in peripheral blood mononuclear cells of patients with chronic obstructive pulmonary disease. Respir Res. 2017;18:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Q, He Y, Kan W, et al. microRNA‐32‐5p targets KLF2 to promote gastric cancer by activating PI3K/AKT signaling pathway. Am J Transl Res. 2019;11(8):4895‐4908. [PMC free article] [PubMed] [Google Scholar]
- 12. Karimi N, Ali Hr FM, Safaralizadeh R, et al. Serum overexpression of miR‐301a and miR‐23a in patients with colorectal cancer. J Chin Med Assoc. 2019;82(3):215‐220. [DOI] [PubMed] [Google Scholar]
- 13. Zhang Y, Lin C, Liao G, et al. MicroRNA‐506 suppresses tumor proliferation and metastasis in coloncancer by directly targeting the oncogene EZH2. Oncotarget. 2015;6(32):32586‐32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo S, Yang P, Jiang X, et al. Genetic and epigenetic silencing of microRNA‐506‐3p enhances COTL1oncogene expression to foster non‐small lung cancer progression. Oncotarget. 2017;8(1):644‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zu C, Liu T, Zhang G. MicroRNA‐506 inhibits malignancy of colorectal carcinoma cells by targeting LAMC1. Ann Clin Lab Sci. 2016;46(6):666‐674. [PubMed] [Google Scholar]
- 16. Sun Y, Hu L, Zheng H, et al. miR‐506 inhibits multiple targets in the epithelial‐to‐mesenchymal transition network and is associated with good prognosisin epithelial ovarian cancer. J Pathol. 2015;235(1):25‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wen SY, Lin Y, Yu YQ, et al. miR‐506 acts as atumor suppressor by directly targeting the hedgehog pathway transcription factor Gli3 in human cervical cancer. Oncogene. 2015;34(6):717‐725. [DOI] [PubMed] [Google Scholar]
- 18. Chen J, Yu Y, Li S, et al. MicroRNA‐30a ameliorates hepatic fibrosis by inhibiting Beclin1‐mediated autophagy. J Cell Mol Med. 2017;21(12):3679‐3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu H, Wu H, Liu X, et al. Regulation of autophagy by a beclin 1‐targeted microRNA, miR‐30a, in cancer cells. Autophagy. 2009;5(6):816‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yan L, Hu F, Yan X, et al. Inhibition of microRNA‐155 ameliorates experimental autoimmune myocarditis by modulating Th17/Treg immune response. J Mol Med (Ber). 2016;94(9):1063‐1079. [DOI] [PubMed] [Google Scholar]
- 21. Zhong KY, Li ER, Zhang ZW, et al. Value of serum miR‐21 and miR‐155 in diagnosis and prognosis evaluation of acute renal injury. Chin J lmmunol. 2018;34(12):107‐111. [Google Scholar]
- 22. Zhang H, Zhao Z, Pang X, et al. Genistein protects against Ox‐LDL‐induced inflammation through microRNA‐155/SOcS1‐mediated repression of NF‐KB signaling pathway in HUVECs. Inflammation. 2017;40(4):1450‐1459. [DOI] [PubMed] [Google Scholar]
- 23. Ma L, Xue H, Wang F, et al. microRNA‐155 may be involved in the pathogenesis of atopic dermatitis by modulating the differentiation and function of T helper type 17 (Th17) cells. Clin Exp lmmunol. 2015;181(1):142‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Y, Feng T, Duan S, et al. miR‐155 promotes fibroblast‐likesynoviocyte proliferation and inflammatory cytokine secretionin rheumatoid arthritis by targeting FOXO3a. Exp Ther Med. 2020;19(2):1288‐1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang M, Wang L, Zhang X, et al. Overexpression of miR‐31 inperipheral blood mononuclear cells (PBMC) from patients with ankylosing spondylitis. Med Sci Monit. 2017;23:5488‐5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sheng B, Li S, Yuan X, Chai L, Cao C. The association between epidermal growth factor and the treatment of deep second degree burn wounds: a meta‐analysis. Int J Clin Exp Med. 2017;10(7):9871‐9876. [Google Scholar]
- 27. Tan L, Hou Z, Gao Y. Efficacy of combined treatment with vacuum sealing drainage and recombinant human epidermal growth factor for refractory wounds in the extremities and its effect on serum levels of lL‐6TNF‐a and IL‐21J1. Exp Ther Med. 2018;15(1):288‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hawkes JE, Nguyen GH, Fujita M, et al. microRNAs in psoriasis. J Invest Dermatol. 2016;136(2):365‐371. [DOI] [PubMed] [Google Scholar]
- 29. Liu B, Guo Z, Gao W. miR‐181b‐5p promotes proliferation and inhibits apoptosis of hypertrophic scar fibroblasts through regulating the MEK/ERK/p21 pathway. Exp Ther Med. 2019;17(3):1537‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cao C, Wang W, Lu L, et al. Inactivation of Beclin‐1‐dependent autophagy promotes ursolic acid‐induced apoptosis in hypertrophic scar fibroblasts. Exp Dermatol. 2018;27(1):58‐63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets that have been used and/or analyzed during the current study are available from the corresponding author upon reasonable request.


