Figure 1.
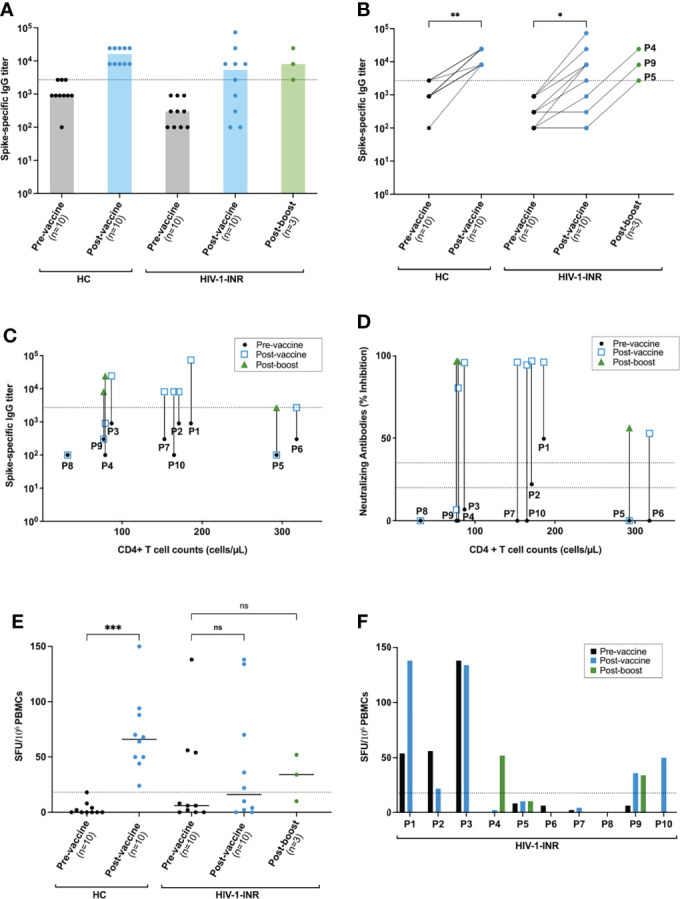
Spike-specific anti-SARS-CoV-2 antibody and cellular responses in HIV-INR and healthy donors after mRNA-based COVID vaccination. (A, B) IgG Spike-specific titers determined by ELISA for both of the 2 cohorts: healthy controls (HC) and HIV-INR at the 3 defined time-points: pre-vaccination (pre-vaccine), post-vaccination (post-vaccine) and, for the HIV-INR non-responder patients, 3 w post boost vaccination (post-boost). IgG Spike-specific titers of the HIV-INR (C) and individual inhibition percentages (% IH) against RBD determined by NeutraLISA (D) of all the HIV-INR patients taking into account their individual CD4+ T cell counts at the 3 different time-points. (E) IFN-γ ELISpot values (SFU per 106 PBMCs) for all healthy individuals (HC) and HIV-INR patients at the 3 different time-points after in vitro stimulation with overlapping Spike peptides of SARS-CoV-2. (F) IFN-γ ELISpot values for individual patients. Differences between the groups were calculated using Mann–Whitney test or Kruskal-Wallis test for comparison of two groups. Non-significant differences were indicated as “ns”. P-values below 0.05 were considered significant and were indicated by asterisks: *p< 0.05; **p < 0.01; ***p < 0.001.
