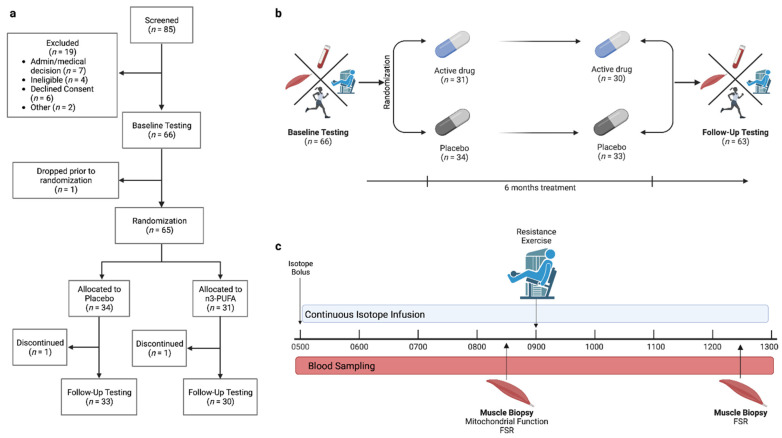
Figure 1.
Participant enrollment and study design. (a) Enrollment numbers and flow chart for the study. (b) Overall study design of the intervention, which included baseline and follow-up assessments of physical activity, cardiorespiratory fitness, muscle strength and function, mitochondrial physiology, body composition, and whole-body and mixed muscle protein synthesis. All assessments were completed before and after 6 months of placebo or n3-PUFA supplementation, administered in a randomized, double-blind design. (c) Study design for the inpatient study day. Figure created using BioRender.com.