Figure 3. Serious Adverse Events in a Study of 5-Year Outcomes in Patients With Fully Magnetically Levitated vs Axial-Flow Left Ventricular Assist Devices .
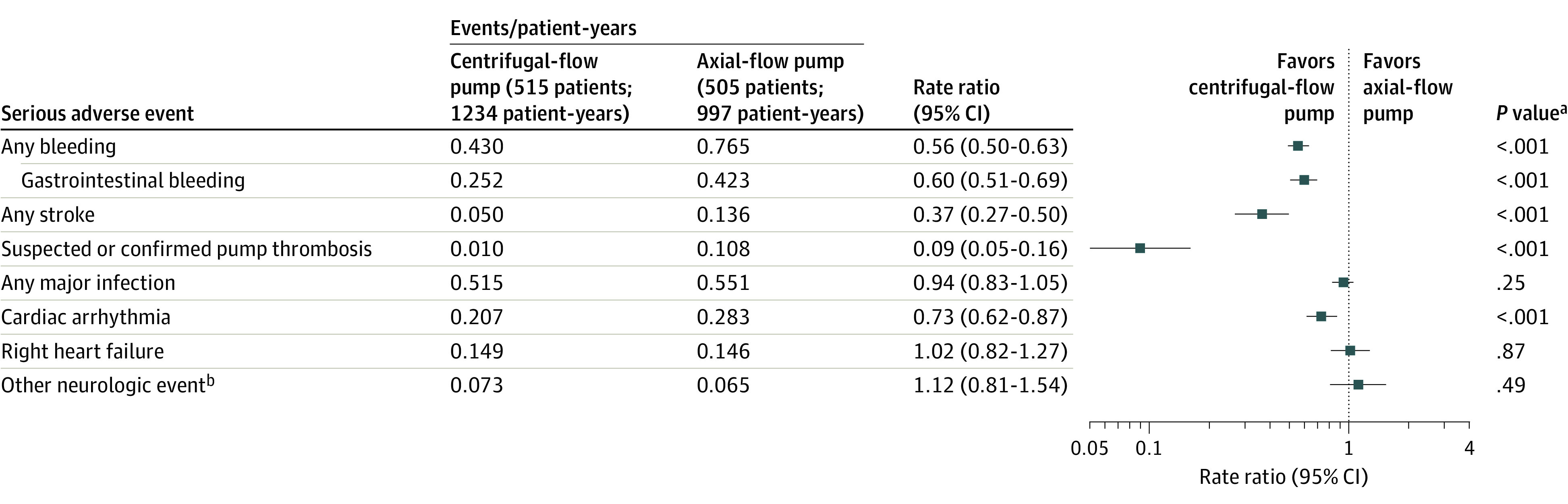
Serious adverse events are defined as those causing death or congenital abnormality or birth defect or a life-threatening illness or injury that results in permanent disability, requires hospitalization, or prolongs a hospitalization and/or requires intervention to prevent permanent injury or damage. Serious adverse events occurring after 2 years were collected in 178 patients in the magnetically levitated centrifugal-flow pump group and 117 patients in the axial-flow pump group. Other secondary end points are shown in eTable 6 and 7 in Supplement.
aRate ratios and P value from Poisson regression.
bIncludes encephalopathy, seizure, transient ischemic attack, and other neurological events other than stroke.
