Figure 2.
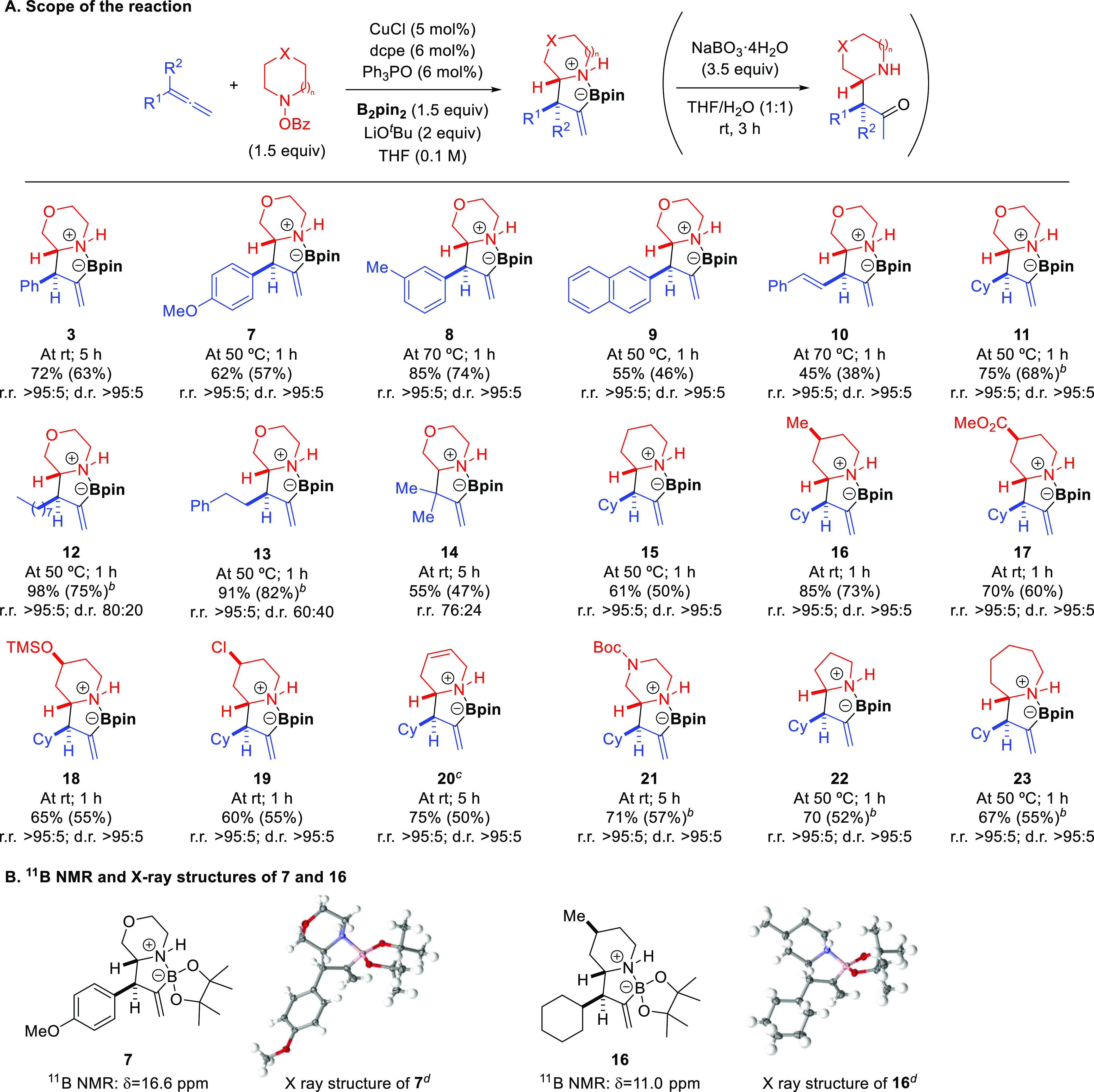
(A) Scope of the copper-catalyzed borylative α-allylation of O-benzoyl cyclic hydroxylamines. aReactions run on a 0.3 mmol scale. Regioisomeric ratios (r.r.; C–C vs C–N coupling) and diastereomeric ratios (d.r.) were determined by 1H NMR analysis. Yields of isolated products are shown in brackets. bThose values refer to the oxidized product. cSynthesized from 4-(acryloyloxy)piperidin-1-yl benzoate. (B) dEllipsoids shown at 50% probability.
