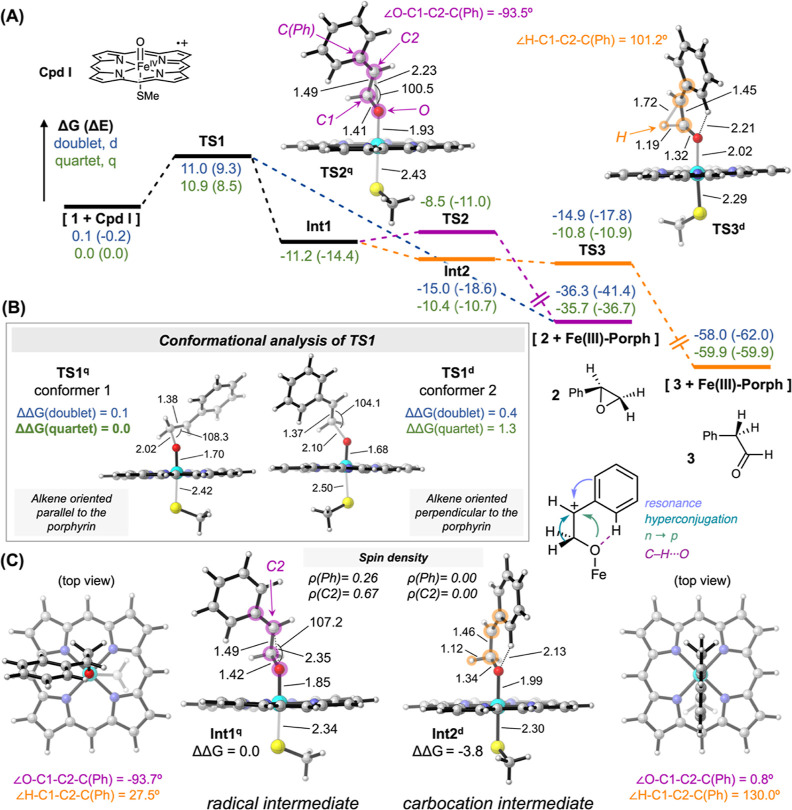
Figure 2.
Enzyme-free model DFT calculations. (A) DFT-calculated competing reaction pathways for styrene (1) epoxidation (purple pathway) and carbonyl formation (orange pathway) catalyzed by the iron-oxo heme enzyme-free computational model. Relative Gibbs energies (ΔG) and electronic energies (ΔE, in parenthesis) are reported, in blue for the doublet electronic state (d) and in green for the quartet electronic state (q). Lowest-energy DFT-optimized key transition state geometries are shown. See Figure S1 for additional details on the intrinsic mechanisms. (B) Conformational analysis of TS1. (C) Lowest-energy DFT-optimized structures of key covalent radical and carbocation intermediates (Int1 and Int2, respectively). Energies, distances, angles, and spin density values are given in kcal·mol–1, angstroms (Å), degrees (°), and atomic units (a.u.), respectively.