Abstract
Aims
The aims of this study is to assess by an updated meta-analysis the clinical outcomes related to permanent pacemaker implantation (PPI) after transcatheter aortic valve implantation (TAVI) at long-term (≥12 months) follow-up (LTF).
Methods and results
A comprehensive literature research was performed on PubMed and EMBASE. The primary endpoint was all-cause death. Secondary endpoints were rehospitalization for heart failure, stroke, and myocardial infarction. A subgroup analysis was performed according to the Society of Thoracic Surgeon—Predicted Risk of Mortality (STS-PROM) score. This study is registered with PROSPERO (CRD42021243301). A total of 51 069 patients undergoing TAVI from 31 observational studies were included. The mean duration of follow-up was 22 months. At LTF, PPI post-TAVI was associated with a higher risk of all-cause death [risk ratio (RR) 1.18, 95% confidence interval (CI) 1.10–1.25; P < 0.001] and rehospitalization for heart failure (RR 1.32, 95% CI 1.13–1.52; P < 0.001). In contrast, the risks of stroke and myocardial infarction were not affected. Among the 20 studies that reported procedural risk, the association between PPI and all-cause death risk at LTF was statistically significant only in studies enrolling patients with high STS-PROM score (RR 1.25, 95% CI 1.12–1.40), although there was a similar tendency of the results in those at medium and low risk.
Conclusion
Patients necessitating PPI after TAVI have a higher long-term risk of all-cause death and rehospitalization for heart failure as compared to those who do not receive PPI.
Keywords: Permanent pacemaker implantation, Transcatheter aortic valve implantation, Transcatheter aortic valve replacement, Clinical outcome, Personalized medicine, Meta-analysis
Graphical Abstract
Graphical Abstract.
What’s new?
Since the first clinical report in 2002, transcatheter aortic valve implantation (TAVI) has emerged as a worthy, less-invasive, and safe alternative for the therapeutic management of patients with severe aortic stenosis (AS).1 Over the years, TAVI gained the role of treatment of choice in inoperable patients and those at high or intermediate surgical risk.2 More recently, two randomized controlled clinical trials (RCTs) have supported the indication for TAVI even in patients at low surgical risk.3,4
Transcatheter aortic valve implantation (TAVI), compared with surgery, led to an increased need for post-operative permanent pacemaker implantation (PPI).
In this meta-analysis including 51 069 patients across 31 observational studies, PPI post-TAVI was associated with an increased long-term risk of all-cause death and rehospitalization for heart failure.
These results help to characterize the prognosis of patients undergoing TAVI.
Introduction
Among different complications that can occur after TAVI, the development of conduction abnormalities is extremely frequent.5 An injury to the atrioventricular conduction system during balloon valvuloplasty or prosthesis implantation and ischaemia of the conduction pathways can lead to advanced conduction disorders that often require permanent pacemaker implantation (PPI).6
The incidence of post-procedural PPI is higher after TAVI compared with surgical aortic valve replacement (SAVR); in particular, a recent RCT showed that PPI occurred in 33% of TAVI and 20% of SAVR patients at 5 years of follow-up.7
The need for PPI in patients undergoing TAVI is known to be influenced by both clinical and technical aspects.8 However, to date, the prognostic impact of PPI after TAVI is still debated; indeed, recent observational data have reported conflicting results.9–13 Furthermore, previous meta-analyses (with a small number of studies included, different adjudication methods of the outcome of interest, and short-term follow-up period) have yielded jarring results.14–19
On such bases, we performed the present systematic review and meta-analysis to evaluate the impact of PPI on long-term clinical outcomes of patients with AS undergoing TAVI.
Methods
This meta-analysis was carried out in accordance with the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines20 and was registered within the PROSPERO International Prospective Register of Systematic Reviews (CRD42021243301).
Search strategy and selection criteria
A systematic and comprehensive literature research was performed on PubMed and EMBASE databases, from inception to October 2021, to identify studies that investigated the impact of PPI after TAVI on clinical outcomes. We used a combination of the following keywords and MeSH terms: TAVI, PPI, mortality. The full research strategy is listed in Supplementary material online, Table S1.
All records retrieved from the research were systematically screened in parallel and independently by two authors (A.Z. and G.P.), according to titles and abstracts; conflicts were resolved by collegial discussion.
The following inclusion criteria were used: (i) studies reporting adverse events related to PPI after TAVI in native valves and (ii) studies reporting long-term clinical outcomes (follow-up ≥ 12 months).
We excluded studies in which outcomes of interest were not clearly reported or were impossible to extract from the published results, studies that included patients with pacemaker before TAVI, conference abstracts, comments, editorials, case reports, systematic reviews, and meta-analysis.
When two or more studies were reported from the same cohort of subjects, the most recent publication or the one with the longest follow-up was included in the analysis.
Data extraction and quality assessment
Data extraction from the studies included was performed independently by two coauthors (A.Z. and G.P.) using a standardized worksheet. If available, the following items were collected: first author’s name, year of publication, study design, region, number of centres where the study was carried out, sample size, incidence of PPI, timing of PPI, baseline demographic and clinical characteristics of the population, valve type implanted, Society of Thoracic Surgeons—Predicted Risk of Mortality (STS-PROM) score,21 and follow-up duration.
Particularly, the STS-PROM score is a validated risk prediction model based on ∼50 clinical pre-operative variables from the STS National Adult Cardiac Surgery Database such as age, race, cardiovascular risk factors, clinical presentation; it allows to calculate a patient’s risk of mortality for both the most commonly performed cardiac surgeries and TAVI.
Finally, the data necessary for the outcome analysis were also extracted; data at 1-year follow-up that were not directly available were retrieved from another meta-analysis that previously retrieved data from the corresponding author.14
Quality assessment of the studies was made independently by two coauthors (A.Z. and G.P.) using the standardized Newcastle–Ottawa Scale (NOS),22 producing a quality score (from 0 to 9) for each study included.
Study endpoints
The primary endpoint of the study was all-cause death at long-term (≥ 12 months) follow-up (LTF). The risk of all-cause death was calculated at 30 days and 1 year to assess the possible impact of follow-up duration.
Secondary endpoints were rehospitalization for heart failure, stroke, and myocardial infarction at LTF and 1 year.
Statistical analysis
Categorical dichotomous data were summarized across treatment arms, compared, and reported as crude risk ratio (RR) with the corresponding 95% confidence interval (CI). As primary analysis, we used DerSimonian and Laird random-effects model. As secondary analyses, we also reported effects estimates as crude odds ratio (OR) with the corresponding 95% CI and computed Mantel–Haenszel fixed-effects model. We evaluated heterogeneity of effects using the Cochran Q test statistic and Higgins and Thompson I2. According to prespecified cutoffs, low heterogeneity was defined as an I2 <25%, moderate heterogeneity as an I2 between 25% and 75%, and high heterogeneity as an I2 >75%. We visually inspected funnel plots for asymmetry and used Egger’s regression asymmetry test to assess the potential effect of publication bias. Furthermore, we performed a subgroup analysis stratifying studies according to the mortality risk of patients predicted by the STS-PROM score [high risk of mortality (≥8%), intermediate risk (4–8%), and low risk (<4%), as previously reported]23 to evaluate whether the impact of PPI after TAVI on all-cause death at LTF was influenced by this variable.
Sensitivity analyses were performed by comparing the results of the primary and secondary analyses. In order to investigate potential sources of heterogeneity for the LTF outcomes, we performed several univariable random-effects meta-regression analyses with the DerSimonian and Laird method according to age, sex category, atrial fibrillation, diabetes mellitus, coronary artery disease (CAD), left ventricular ejection fraction (LVEF), number of self-expanding and balloon-expanding valves implanted, NOS, and duration of follow-up. Secondary and subgroup analyses were not prespecified. Descriptive characteristics were presented as mean ± standard deviation or median (inter-quartile range) for continuous variables and as frequencies and percentages for categorical variables. Statistical analysis was performed using Stata 17 (StataCorp).
Results
The initial search retrieved 2066 records (616 from PubMed and 1450 from EMBASE). 2003 records were excluded because of different study design or topic of interest after the evaluation of titles and abstracts. Then, other 63 records were subsequently excluded after full-text assessment. Finally, 31 records were selected (Supplementary material online, Table S2), with 51 069 patients undergoing TAVI included in the analysis. The mean follow-up duration was of 22 months (range 12–60 months).
Study and population characteristics are summarized in Table 1 and Supplementary material online, Table S3.
Table 1.
Main characteristics of the studies included in the systematic review
| Author | Year | Type of study | Region | Centres | Inclusion period | No. of patients | No. of patients undergoing PPI (%) | Timing of PPI | Type of valve implanted (%) | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Alasti et al.24 | 2018 | Obs, prospective | Australia | 1 | April 2012–October 2016 | 152 | 38 (25.0) | Within 30 days | MEV (100) | 12 |
| Aljabbary et al.25 | 2018 | Obs, retrospective | Canada | 10 | April 2010–October 2015 | 1263 | 186 (14.7) | During hospitalization | NA | 33 |
| Ashraf et al.12 | 2020 | Obs, retrospective | Arizona | 1 | January 2012–July 2018 | 243 | 22 (9.1) | Within 30 days | BEV (100) | 36 |
| Biner et al.26 | 2014 | Obs, retrospective | Israel | 1 | NA | 230 | 58 (25.4) | NA | SEV (87.4) | 19.5 |
| BEV (12.6) | ||||||||||
| Buellesfeld et al.27 | 2012 | Obs, prospective | Switzerland, Germany | 2 | August 2007–March 2010 | 305 | 98 (32.1) | Within 30 days | SEV (89.5) | 12 |
| BEV (10.5) | ||||||||||
| Chamandi et al.28 | 2018 | Obs, prospective | International | 9 | May 2007–February 2011 | 1629 | 322 (19.8) | Within 30 days | SEV (53.9) | 52 |
| BEV (43.8) | ||||||||||
| Costa et al.10 | 2019 | Obs, prospective | Italy | 1 | June 2007–February 2018 | 1116 | 145 (13.0) | Within 30 days | SEV (72.5) | 12 |
| BEV (27.2) | ||||||||||
| D’Ancona et al.29 | 2011 | Obs, prospective | Germany | 1 | April 2008–March 2011 | 322 | 20 (6.2) | Within 30 days | BEV (100) | 12 |
| De Carlo et al.30 | 2011 | Obs, prospective | Italy | 3 | September 2007–July 2010 | 275 | 66 (24.0) | 0–2 days | SEV (100) | 12 |
| Du et al.11 | 2019 | Obs, retrospective | China | 1 | March 2013–October 2018 | 256 | 38 (14.8) | Within 30 days | SEV (100) | 12 |
| Engborg et al.31 | 2016 | Obs, prospective | Denmark | 1 | March 2008–September 2012 | 128 | 41 (32.0) | Within 30 days | SEV (78.1) | 46.2 |
| BEV (21.9) | ||||||||||
| Fadahunsi et al.32 | 2016 | Obs, retrospective | USA | 220 | November 2011–September 2014 | 9785 | 651 (6.7) | Within 30 days | SEV (11.2) | 12 |
| BEV (88.8) | ||||||||||
| Fujita et al.33 | 2019 | Obs, prospective | Germany | Multicentre | 2011–15 | 20 872 | 3459 (16.6) | During hospitalization | SEV (36.0) | 12 |
| BEV (53.7) | ||||||||||
| DFM (1.5) | ||||||||||
| Gensas et al.34 | 2014 | Obs, retrospective | Brazil | 18 | January 2008–February 2012 | 353 | 89 (25.2) | Within 30 days | SEV (85.8) | 60 |
| BEV (14.2) | ||||||||||
| Giustino et al.35 | 2016 | Obs, retrospective | Europe | 4 | November 2005–December 2011 | 947 | 145 (13.2) | Within 30 days | SEV (52.1) | 60 |
| BEV (47.9) | ||||||||||
| Gonska et al.36 | 2018 | Obs, retrospective | Germany | 1 | February 2014–September 2016 | 612 | 168 (27.5) | NA | SEV (4.4) | 12 |
| BEV (58.8) | ||||||||||
| MEV (36.8) | ||||||||||
| Houthuizen et al.37 | 2012 | Obs, prospective | Netherlands | 8 | November 2005–December 2010 | 797 | 118 (14.8) | Within 30 days | SEV (61.4) | 15 |
| BEV (38.6) | ||||||||||
| Jørgensen et al.38 | 2019 | Obs, prospective | Denmark | 1 | 2007–17 | 816 | 132 (16.2) | Within 30 days | SEV (82.6) | 30 |
| BEV (9.4) | ||||||||||
| MEV (8.0) | ||||||||||
| Kostopoulou et al.39 | 2015 | Obs, prospective | Greece | 1 | January 2010–February 2012 | 45 | 10 (22.2) | Within 30 days | SEV (100) | 24 |
| López-Aguilera et al.40 | 2018 | Obs, prospective | Spain | 1 | April 2008–December 2015 | 217 | 39 (15.0) | During hospitalization | SEV (100) | 37 |
| Meduri et al.9 | 2019 | Obs, prospective | North America, Europe, Australia | 55 | September 2014–December 2015 | 704 | 245 (34.8) | Within 30 days | SEV (33.8) | 12 |
| MEV (66.2) | ||||||||||
| Mouillet et al.41 | 2015 | Obs, prospective | International | 29 | January 2010–October 2011 | 883 | 252 (30.3) | Within 1 year | SEV (100) | 12 |
| Nadeem et al.42 | 2018 | Obs, retrospective | Ohio | 1 | 2011 – 2017 | 672 | 146 (21.7) | Within 1 year | SEV (55.5) | 12 |
| BEV (44.2) | ||||||||||
| Nazif et al.43 | 2015 | Obs, retrospective | International | 21 | May 2007–September 2011 | 1973 | 173 (8.8) | Within 30 days | BEV (100) | 12 |
| Nijenhuis et al.44 | 2017 | Obs, retrospective | Netherlands | 1 | June 2007–June 2015 | 155 | 37 (23.9) | Within 30 days | NA | 18.6 |
| Pereira et al.45 | 2013 | Obs, retrospective | Portugal | 1 | August 2007–May 2011 | 58 | 19 (32.8) | During hospitalization | SEV (100) | 12 |
| Rogers et al.46 | 2018 | Obs, prospective | USA | 1 | January 2013–December 2015 | 614 | 145 (23.6) | Within 30 days | SEV (22.0) | 12 |
| BEV (78.0) | ||||||||||
| Rück et al.13 | 2021 | Obs, population-based cohort | Sweden | 8 | January 2008–December 2018 | 3420 | 481 (14.1) | Within 30 days | BEV (38.4) | 32.4 (20.4 m for HF outcome) |
| Schymik et al.47 | 2015 | Obs, retrospective | Germany | 1 | May 2008–April 2012 | 634 | 69 (10.8) | Within 24 h | SEV (19.2) | 12 |
| BEV (80.8) | ||||||||||
| Urena et al.48 | 2014 | Obs, retrospective | International | 8 | January 2005–February 2013 | 1556 | 239 (15.4) | Within 30 days | SEV (44.9) | 22 |
| BEV (55.1) | ||||||||||
| Walther et al.49 | 2018 | Obs, prospective | Europe, Australia | 12 | December 2011–September 2015 | 198 | 29 (14.7) | During hospitalization | SEV (100) | 12 |
BEV, balloon-expanding valves; HF, heart failure; MEV, mechanically expandable valves; NA, not available; Obs, observational; PPI, permanent pacemaker implantation; SEV, self-expandable valves.
All studies were of observational nature, of which 16 were prospective and 15 were retrospective.
The indication for PPI varied between studies. PPI was defined as post-procedural or within 30 days after TAVI in most studies; however, some studies also included a minority of patients who experienced PPI after 30 days from the procedure.
The incidence of PPI ranged from 6.2% to 34.8% across the studies.
All-cause death at long-term follow-up
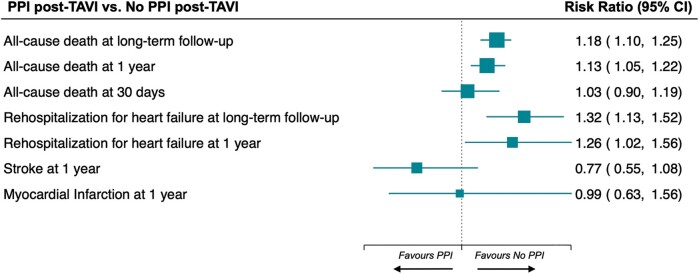
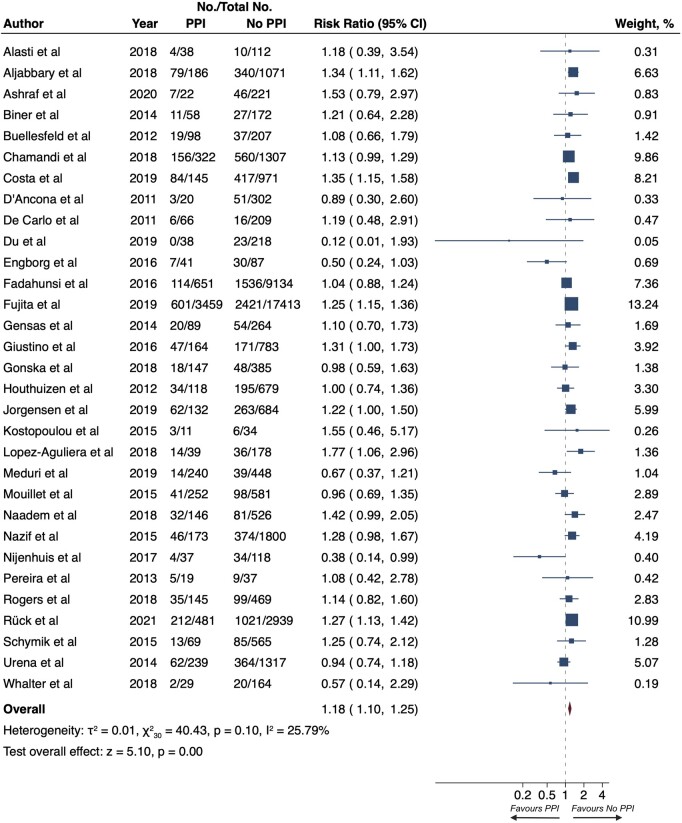
Among 51 069 patients undergoing TAVI, the risk of all-cause death at LTF was higher for patients who experienced PPI (22.9% vs. 19.6%; RR 1.18, 95% CI 1.10–1.25, P < 0.001; Figure 1). The heterogeneity between the studies was moderate (I2 = 25.79%) and there was a potential publication bias detected by the Egger regression and funnel plot inspection (P = 0.015; Supplementary material online, Figure S5).
Figure 1.
Risk of all-cause death at long-term follow-up. Squares represent risk ratios, with the size of the squares indicating weight of the studies and horizontal lines representing 95% CIs. The diamond represents the pooled risk ratio with the points of the diamond representing 95% CIs. CIs, confidence intervals.
In the subgroup analysis performed in the 20 studies reporting the mortality risk of patients predicted by the STS-PROM score, the association between PPI and all-cause death risk at LTF was significant only in studies enrolling patients with high STS-PROM score (26.7% vs. 24.6%; RR, 1.25; 95% CI, 1.12–1.40; Supplementary material online, Figure S1), but not in those enrolling patients at intermediate (19.9% vs. 17.3%; RR, 1.11; 95% CI, 0.98–1.25; Supplementary material online, Figure S1) or low risk (47.0% vs. 38.5%; RR, 1.22; 95% CI, 1.00–1.50; Supplementary material online, Figure S1). It is worth noting, however, that the tendency of the results was similar in all three groups without a significant heterogeneity (P = 0.33; Supplementary material online, Figure S1).
All-cause death at 1 year and 30 days
In a pooled analysis of 45 270 patients, those with PPI post-TAVI experienced an increased risk of all-cause death at 1 year (16.6% vs. 15.1%; RR 1.13, 95% CI 1.05–1.22; P < 0.001; Supplementary material online, Figure S2). There was low heterogeneity between studies (I2 = 8.37%) and a potential publication bias (P = 0.015; Supplementary material online, Figure S5). Conversely, the risk of all-cause death at 30 days, pooled from 40 806 patients, was not different between patients with PPI and without it (3.7% vs. 3.9%; RR 1.03, 95% CI 0.90–1.19; P = 0.66; Supplementary material online, Figure S3). The heterogeneity across the studies was low (I2 = 0.00%) and no potential publication bias was detected (P = 0.334; Supplementary material online, Figure S5).
Rehospitalization for heart failure at long-term follow-up and 1 year
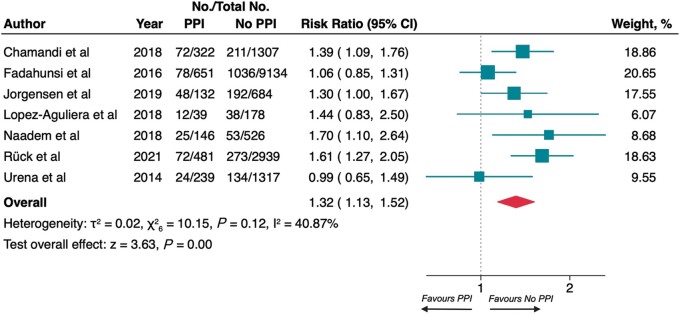
The pooled results among 18 095 patients demonstrated that PPI was associated with rehospitalization for heart failure at LTF (16.5% vs. 12.0%; RR 1.32, 95% CI 1.13–1.52; P < 0.001; Figure 2). The heterogeneity across the studies was moderate (I2 = 40.87%) and no significant asymmetry was detected (P = 0.752; Supplementary material online, Figure S5).
Figure 2.
Risk of rehospitalization for heart failure at long-term follow-up. Squares represent risk ratios, with the size of the squares indicating weight of the studies and horizontal lines representing 95% CIs. The diamond represents the pooled risk ratio with the points of the diamond representing 95% CIs. CIs, confidence intervals.
The same results were detected at 1 year (12.2% vs. 10.7%; RR 1.26, 95% CI 1.02–1.56; P = 0.03; Supplementary material online, Figure S4) among 14 867 patients; the heterogeneity was moderate (I2 = 42.48%), no potential publication bias was disclosed (P = 0.766; Supplementary material online, Figure S5).
Stroke at 1 year and myocardial infarction at 1 year
All studies reporting data regarding stroke and myocardial infarction in the LTF had 1 year observation time so that both endpoint evaluations at LTF and 1 year were coincident.
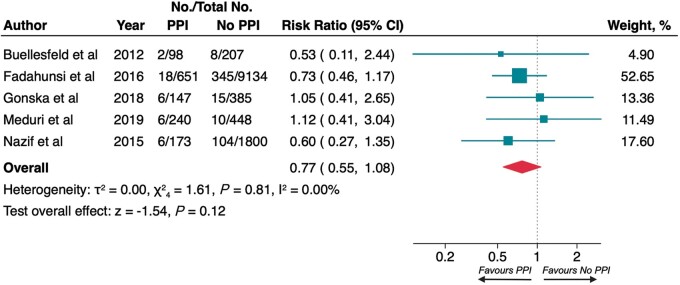
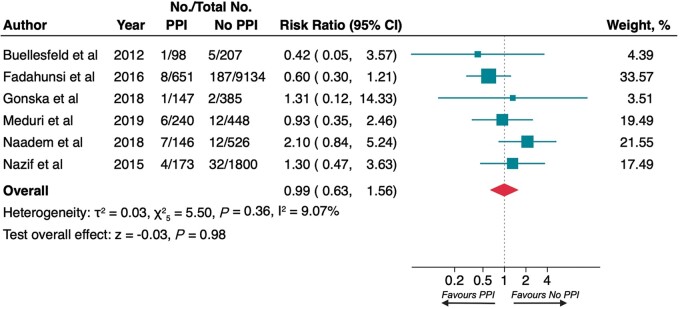
At 1 year, no difference in risk of stroke (2.9% vs. 4.0%; RR 0.77, 95% CI 0.55–1.08; P = 0.12; Figure 3) and myocardial infarction (1.9% vs. 2.0%; RR 0.99, 95% CI 0.63–1.56; P = 0.98; Figure 4) was observed between patients who required PPI and controls. The heterogeneity between studies was low (I2 0.00% and 9.07%, respectively) and there was not significant publication bias (P = 0.383, P = 0.980; Supplementary material online, Figure S5).
Figure 3.
Risk of stroke at 1 year. Squares represent risk ratios, with the size of the squares indicating weight of the studies and horizontal lines representing 95% CIs. The diamond represents the pooled risk ratio with the points of the diamond representing 95% CIs. CIs, confidence intervals.
Figure 4.
Risk of myocardial infarction at 1 year. Squares represent risk ratios, with the size of the squares indicating weight of the studies and horizontal lines representing 95% CIs. The diamond represents the pooled risk ratio with the points of the diamond representing 95% CIs. CIs, confidence intervals.
Sensitivity and meta-regression analyses
Sensitivity analyses performed comparing primary and secondary analyses obtained similar results (Supplementary material online, Table S5).
Meta-regression analyses showed no significant relation between most covariates and long-term outcomes. However, NOS was inversely associated with a higher risk of rehospitalization for heart failure at LTF related to PPI.
Discussion
The need for post-procedural PPI represents the Achille’s heel of TAVI. In this meta-analysis of 31 observational studies, we found that patients who underwent PPI post-TAVI had a greater risk of all-cause death and rehospitalization for heart failure at 1 year and long-term follow-up.
Previous meta-analyses were contradictory about the relationship between PPI post-TAVI and the risk of worse clinical outcomes: indeed, some showed a significant impact in hard clinical endpoints such as all-cause death14,18 and rehospitalization for heart failure,14 while most did not show a significant clinical worsening;15–17 of note, the follow-up period was mostly limited to 1 year.
To the best of our knowledge, this is the most updated meta-analysis, with the largest sample size that evaluates clinical outcomes at various follow-up times (including long-term follow-up); moreover, this is the first article performing a subgroup analysis according to the preoperative procedural risk.
The ventricular dyssynchrony related to the right ventricular pacing could play an important role in increasing the risk of all-cause death among patients with PPI.50–52 Furthermore, it might also explain the increased risk of rehospitalization for heart failure and the absence of an impact of PPI on short-term mortality (at 30 days). In this regard, Nadeem et al.42 documented that patients with right ventricular pacing >40% had a higher risk of heart failure compared with those who experienced a lower right ventricular pacing burden.
Unfortunately, the few data and the variable pacing percentage cut-offs adopted in the various studies did not allow to perform a pooled analysis to evaluate the influence of the aforementioned variable on clinical outcomes. By this logic, different types of ventricular pacing (such as cardiac resynchronization therapy or His pacing) or proper device programming, could have a beneficial impact on the prognosis of patients undergoing post-procedural PPI.
Furthermore, higher mortality observed in patients who underwent PPI after TAVI may be related to different causes, both cardiac and non-cardiac, so that PPI could represent only a simple bystander. For instance, worse outcomes related to PPI could also be explained by the mechanical or ischaemic injury to the conduction system that can occur during TAVI. Indeed, new-onset persistent left bundle branch block was found to be associated with an increased risk of all-cause death and rehospitalization for heart failure.14
Interestingly, the significantly higher risk of all-cause death at LTF associated with PPI was confined to studies enrolling patients at high preoperative risk of mortality (≥8%, according to the STS-PROM score), while it was of borderline significance in those enrolling patients at medium or low risk. These findings are probably affected by the greater multimorbidity burden of high-risk patients undergoing TAVI and, therefore, by the relatively short follow-up available; indeed, among patients with a lower multimorbidity burden, a longer follow-up would be needed to establish the association between PPI and long-term mortality. On the other hand, the benefit of TAVI over SAVR regarding procedural risks is probably smaller in the group with STS-PROM score < 8% and so, in these patients, the disadvantage related to the long-term impact of PPI may be larger. Probably, with the extension of the indications for TAVI also in low-risk patients, the recruitment of patients with a lower average age will help to highlight this issue. However, it should be emphasized that the STS-PROM score may not intercept all comorbidities that could impact long-term mortality and this may limit the interpretation of these results.
Although right ventricular pacing is associated with an increased risk of atrial fibrillation,53 in our study PPI was not associated with a higher risk of stroke at 1-year follow-up. This may be caused by the relatively short follow-up of the studies included and the presence of confounding factors (such as post-procedural atrial fibrillation and antithrombotic therapy) that were not adjusted during the analysis.
Recently, several studies have documented the presence of electrical, anatomical, and procedural predictors of PPI after TAVI such as age, pre-existing conduction abnormalities, calcification of the left ventricular outflow tract, the use of self-expanding valve type, balloon valvuloplasty, and valve implantation depth.54 Other factors were found to predict a high percentage of long-term pacing in patients who experienced post-TAVI PPI such as high left ventricular outflow tract diameter ratio, high aortic annulus diameter ratio, new onset of left bundle branch block, time to PPI >2 days, and therapy with beta-blockers.55 Consequently, the choice of intervention modality in patients with AS should take into account the factors mentioned above.
In light of the results of this meta-analysis, strategies aimed to reduce the incidence of PPI might have an impact on the long-term outcomes of patients undergoing TAVI. Recently, higher valve implantation showed a reduction in conduction abnormalities and permanent pacemaker requirement, without compromising procedural safety or valve haemodynamic.56 In addition, other specific changes to the TAVI implementation techniques have been proposed.57,58 Findings from other ongoing trials are needed to strengthen this evidence.
Limitations
Our meta-analysis has some limitations. Since systematic reviews and meta-analyses rely on the quality of included studies, we could only use observational studies, many of them with retrospective follow-up. Besides, the lack of pacing frequency data did not allow us to judge the influence of this variable on outcomes. Also, the lack of single patient-level data regarding the mortality outcome has foreclosed subgroup analyses and the possibility of establishing whether the need for PPI is an independent predictor of worse outcomes. Further, indications for PPI were different in the various studies limiting results reproducibility. Finally, over the period time of the present meta-analysis, there have been some important changes and evolution in the design and technique of TAVI procedure.
Conclusion
Patients who underwent PPI after TAVI had an increased risk of all-cause death and rehospitalization for heart failure 1 year after the implantation and at the long-term follow-up. On the other hand, PPI did not modify the risk of all-cause death after 30 days, stroke, and myocardial infarction.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
A.Z. and F.B. conceived and designed the study. A.Z. and G.P. independently assessed the studies for possible inclusion and collected the data. A.Z. analysed the data. A.Z., G.P., and M.L. produced the first draft of the manuscript. F.B. and F.C. critically revised the manuscript for important intellectual content. All authors revised and approved the final version of the manuscript. A.Z. and F.B. had final responsibility for the decision to submit for publication.
Conflict of interest: C.T. discloses to have been involved in advisory board meetings or having received speaker’s fees from Abbott, Abiomed, and Biotronic. F.B. discloses to have been involved in advisory board meetings or having received speaker’s fees from Medtronic, Abbott, and Abiomed. All other authors declare no competing interests.
Data availability
The data underlying this article are available in the article and in Supplementary material online.
Contributor Information
Andrea Zito, Department of Cardiovascular and Thoracic Sciences, Università Cattolica del Sacro Cuore, L.go A. Gemelli 1, 00168 Rome, Italy.
Giuseppe Princi, Department of Cardiovascular and Thoracic Sciences, Università Cattolica del Sacro Cuore, L.go A. Gemelli 1, 00168 Rome, Italy.
Marco Lombardi, Department of Cardiovascular and Thoracic Sciences, Università Cattolica del Sacro Cuore, L.go A. Gemelli 1, 00168 Rome, Italy.
Domenico D’Amario, Department of Cardiovascular and Thoracic Sciences, Università Cattolica del Sacro Cuore, L.go A. Gemelli 1, 00168 Rome, Italy; Department of Cardiovascular Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Rocco Vergallo, Department of Cardiovascular and Thoracic Sciences, Università Cattolica del Sacro Cuore, L.go A. Gemelli 1, 00168 Rome, Italy; Department of Cardiovascular Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Cristina Aurigemma, Department of Cardiovascular and Thoracic Sciences, Università Cattolica del Sacro Cuore, L.go A. Gemelli 1, 00168 Rome, Italy; Department of Cardiovascular Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Enrico Romagnoli, Department of Cardiovascular and Thoracic Sciences, Università Cattolica del Sacro Cuore, L.go A. Gemelli 1, 00168 Rome, Italy; Department of Cardiovascular Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Gemma Pelargonio, Department of Cardiovascular and Thoracic Sciences, Università Cattolica del Sacro Cuore, L.go A. Gemelli 1, 00168 Rome, Italy; Department of Cardiovascular Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Piergiorgio Bruno, Department of Cardiovascular and Thoracic Sciences, Università Cattolica del Sacro Cuore, L.go A. Gemelli 1, 00168 Rome, Italy; Department of Cardiovascular Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Carlo Trani, Department of Cardiovascular and Thoracic Sciences, Università Cattolica del Sacro Cuore, L.go A. Gemelli 1, 00168 Rome, Italy; Department of Cardiovascular Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Francesco Burzotta, Department of Cardiovascular and Thoracic Sciences, Università Cattolica del Sacro Cuore, L.go A. Gemelli 1, 00168 Rome, Italy; Department of Cardiovascular Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Filippo Crea, Department of Cardiovascular and Thoracic Sciences, Università Cattolica del Sacro Cuore, L.go A. Gemelli 1, 00168 Rome, Italy; Department of Cardiovascular Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
References
- 1. Figulla HR, Franz M, Lauten A.. The history of transcatheter aortic valve implantation (TAVI)—a personal view over 25 years of development. Cardiovasc Revasc Med 2020;21:398–403. [DOI] [PubMed] [Google Scholar]
- 2. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs Jet al. . 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2021;00:1–72. [Google Scholar]
- 3. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo Met al. . Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695–705. [DOI] [PubMed] [Google Scholar]
- 4. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, et al. ; Evolut Low Risk Trial Investigators . Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019;380:1706–15. [DOI] [PubMed] [Google Scholar]
- 5. Khatri PJ, Webb JG, Rodés-Cabau J, Fremes SE, Ruel M, Lau Ket al. . Adverse effects associated with transcatheter aortic valve implantation: a meta-analysis of contemporary studies. Ann Intern Med 2013;158:35–46. [DOI] [PubMed] [Google Scholar]
- 6. Boon RVD, Nuis RJ, Mieghem NV, Jordaens L, Rodés-Cabau J, Domburg RVet al. . New conduction abnormalities after TAVI-frequency and causes. Nat Rev Cardiol 2012;9:454–63. [DOI] [PubMed] [Google Scholar]
- 7. Gleason TG, Reardon MJ, Popma JJ, Deeb GM, Yakubov SJ, Lee JS, et al. ; CoreValve U.S. Pivotal High Risk Trial Clinical Investigators . 5-Year outcomes of self-expanding transcatheter versus surgical aortic valve replacement in high-risk patients. J Am Coll Cardiol 2018;72:2687–96. [DOI] [PubMed] [Google Scholar]
- 8. Auffret V, Puri R, Urena M, Chamandi C, Rodriguez-Gabella T, Philippon Fet al. . Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation 2017;136:1049–69. [DOI] [PubMed] [Google Scholar]
- 9. Meduri CU, Kereiakes DJ, Rajagopal V, Makkar RR, O’Hair D, Linke Aet al. . Pacemaker implantation and dependency after transcatheter aortic valve replacement in the REPRISE III Trial. J Am Heart Assoc 2019;8:e012594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costa G, Zappulla P, Barbanti M, Cirasa A, Todaro D, Rapisarda Get al. . Pacemaker dependency after transcatheter aortic valve implantation: incidence, predictors and long-term outcomes. EuroIntervention 2019;15:875–83. [DOI] [PubMed] [Google Scholar]
- 11. Du F, Zhu Q, Jiang J, Chen H, Liu X, Wang J.. Incidence and predictors of permanent pacemaker implantation in patients who underwent transcatheter aortic valve replacement: observation of a Chinese population. Cardiol 2020;145:27–34. [DOI] [PubMed] [Google Scholar]
- 12. Ashraf H, Fortuin FD, Sweeney J, DeValeria PA, Lanza LA, Ramsay Get al. . Development of advanced conduction disturbances following balloon-expandable transcatheter aortic valve replacement leads to poorer clinical outcomes. J Arrhythm 2020;36:755–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rück A, Saleh N, Glaser N.. Outcomes following permanent pacemaker implantation after transcatheter aortic valve replacement: SWEDEHEART observational study. JACC Cardiovasc Interv 2021;14:2173–81. [DOI] [PubMed] [Google Scholar]
- 14. Faroux L, Chen S, Muntané-Carol G, Regueiro A, Philippon F, Sondergaard Let al. . Clinical impact of conduction disturbances in transcatheter aortic valve replacement recipients: a systematic review and meta-analysis. Eur Heart J 2020;41:2771–81. [DOI] [PubMed] [Google Scholar]
- 15. Mohananey D, Jobanputra Y, Kumar A, Krishnaswamy A, Mick S, White JMet al. . Clinical and echocardiographic outcomes following permanent pacemaker implantation after transcatheter aortic valve replacement: meta-analysis and meta-regression. Circ Cardiovasc Interv 2017;10:e005046. [DOI] [PubMed] [Google Scholar]
- 16. Regueiro A, Altisent OAJ, Trigo MD, Campelo-Parada F, Puri R, Urena Met al. . Impact of new-onset left bundle branch block and periprocedural permanent pacemaker implantation on clinical outcomes in patients undergoing transcatheter aortic valve replacement. Circ Cardiovasc Interv 2016;9:e003635. [DOI] [PubMed] [Google Scholar]
- 17. Ueshima D, Nai Fovino L, Mojoli M, Napodano M, Fraccaro C, Tarantini G.. The interplay between permanent pacemaker implantation and mortality in patients treated by transcatheter aortic valve implantation: a systematic review and meta-analysis. Catheter Cardiovasc Interv 2018;92:E159–67. [DOI] [PubMed] [Google Scholar]
- 18. Xi Z, Liu T, Liang J, Zhou YJ, Liu W.. Impact of postprocedural permanent pacemaker implantation on clinical outcomes after transcatheter aortic valve replacement: a systematic review and meta-analysis. J Thorac Dis 2019;11:5130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ravaux JM, Di Mauro M, Vernooy K, Kats S, Mariani S, Ronco Det al. . Permanent pacemaker implantation following transcatheter aortic valve implantation using self-expandable, balloon-expandable, or mechanically expandable devices: a network meta-analysis. Europace 2021;23:1998–2009. [DOI] [PubMed] [Google Scholar]
- 20. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie Det al. . Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 21. O'Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. ; Society of Thoracic Surgeons Quality Measurement Task Force . The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2-isolated valve surgery. Ann Thorac Surg 2009;88:S23–42. [DOI] [PubMed] [Google Scholar]
- 22. Wells GA, Shea B, O’Connell D, Peterson J, Welch VL, Al E, The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (19 October 2009, date last accessed).
- 23. Kumar A, Sato K, Narayanswami J, Banerjee K, Andress K, Lokhande Cet al. . Current society of thoracic surgeons model reclassifies mortality risk in patients undergoing transcatheter aortic valve replacement. Circ Cardiovasc Interv 2018;11:1–10. [DOI] [PubMed] [Google Scholar]
- 24. Alasti M, Rashid H, Rangasamy K, Kotschet E, Adam D, Alison Jet al. . Long-term pacemaker dependency and impact of pacing on mortality following transcatheter aortic valve replacement with the LOTUS valve. Catheter Cardiovasc Interv 2018;92:777–82. [DOI] [PubMed] [Google Scholar]
- 25. Aljabbary T, Qiu F, Masih S, Fang J, Elbaz-Greener G, Austin PCet al. . Association of clinical and economic outcomes with permanent pacemaker implantation after transcatheter aortic valve replacement. JAMA Netw Open 2018;1:e180088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Biner S, Michowitz Y, Leshem-Rubinow E, Topilsky Y, Ben-Assa E, Shimiaie Jet al. . Hemodynamic impact and outcome of permanent pacemaker implantation following transcatheter aortic valve implantation. Am J Cardiol 2014;113:132–7. [DOI] [PubMed] [Google Scholar]
- 27. Buellesfeld L, Stortecky S, Heg D, Hausen S, Mueller R, Wenaweser Pet al. . Impact of permanent pacemaker implantation on clinical outcome among patients undergoing transcatheter aortic valve implantation. J Am Coll Cardiol 2012;60:493–501. [DOI] [PubMed] [Google Scholar]
- 28. Chamandi C, Barbanti M, Munoz-Garcia A, Latib A, Nombela-Franco L, Gutiérrez-Ibanez Eet al. . Long-term outcomes in patients with new permanent pacemaker implantation following transcatheter aortic valve replacement. JACC Cardiovasc Interv 2018;11:301–10. [DOI] [PubMed] [Google Scholar]
- 29. D'Ancona G, Pasic M, Unbehaun A, Hetzer R.. Permanent pacemaker implantation after transapical transcatheter aortic valve implantation. Interact Cardiovasc Thorac Surg 2011;13:373–6. [DOI] [PubMed] [Google Scholar]
- 30. De Carlo M, Giannini C, Bedogni F, Klugmann S, Brambilla N, De Marco Fet al. . Safety of a conservative strategy of permanent pacemaker implantation after transcatheter aortic CoreValve implantation. Am Heart J 2012;163:492–9. [DOI] [PubMed] [Google Scholar]
- 31. Engborg J, Riechel-Sarup C, Gerke O, Mickley H, Sandgaard NC, Nissen Het al. . Effect of permanent pacemaker on mortality after transcatheter aortic valve replacement. Scand Cardiovasc J 2017;51:40–6. [DOI] [PubMed] [Google Scholar]
- 32. Fadahunsi OO, Olowoyeye A, Ukaigwe A, Li Z, Vora AN, Vemulapalli Set al. . Incidence, predictors, and outcomes of permanent pacemaker implantation following transcatheter aortic valve replacement: analysis from the U.S. Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc Interv 2016;9:2189–99. [DOI] [PubMed] [Google Scholar]
- 33. Fujita B, Schmidt T, Bleiziffer S, Bauer T, Beckmann A, Bekeredjian R, et al. ; GARY Executive Board . Impact of new pacemaker implantation following surgical and transcatheter aortic valve replacement on 1-year outcome. Eur J Cardiothorac Surg 2020;57:151–9. [DOI] [PubMed] [Google Scholar]
- 34. Gensas CS, Caixeta A, Siqueira D, Carvalho LA, Sarmento-Leite R, Mangione JAet al. . Predictors of permanent pacemaker requirement after transcatheter aortic valve implantation: insights from a Brazilian Registry. Int J Cardiol 2014;175:248–52. [DOI] [PubMed] [Google Scholar]
- 35. Giustino G, Boon RVD, Nicolas JD, Dumonteil N, Chieffo A, Jaegere PDet al. . Impact of permanent pacemaker on mortality after transcatheter aortic valve implantation: the PRAGMATIC (Pooled Rotterdam-Milan-Toulouse in Collaboration) Pacemaker substudy. EuroIntervention 2016;12:1185–93. [DOI] [PubMed] [Google Scholar]
- 36. Gonska B, Keßler M, Wöhrle J, Rottbauer W, Seeger J.. Influence of permanent pacemaker implantation after transcatheter aortic valve implantation with new-generation devices. Neth Heart J 2018;26:620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Houthuizen P, Garsse LV, Poels TT, Jaegere PD, Boon RVD, Swinkels BMet al. . Left bundle-branch block induced by transcatheter aortic valve implantation increases risk of death. Circulation 2012;126:720–8. [DOI] [PubMed] [Google Scholar]
- 38. Jørgensen TH, Backer OD, Gerds TA, Bieliauskas G, Svendsen JH, Søndergaard L.. Mortality and heart failure hospitalization in patients with conduction abnormalities after transcatheter aortic valve replacement. JACC Cardiovasc Interv 2019;12:52–61. [DOI] [PubMed] [Google Scholar]
- 39. Kostopoulou A, Karyofillis P, Livanis E, Thomopoulou S, Stefopoulos C, Doudoumis Ket al. . Permanent pacing after transcatheter aortic valve implantation of a CoreValve prosthesis as determined by electrocardiographic and electrophysiological predictors: a single-centre experience. Europace 2016;18:131–7. [DOI] [PubMed] [Google Scholar]
- 40. López-Aguilera J, Segura Saint-Gerons JM, Sánchez Fernández J, Mazuelos Bellido F, Pan Álvarez-Ossorio M, Suárez De Lezo Jet al. . Long-term clinical impact of permanent cardiac pacing after transcatheter aortic valve implantation with the CoreValve prosthesis: a single center experience. Europace 2018;20:993–1000. [DOI] [PubMed] [Google Scholar]
- 41. Mouillet G, Lellouche N, Yamamoto M, Oguri A, Dubois-Rande JL, Belle EVet al. . Outcomes following pacemaker implantation after transcatheter aortic valve implantation with CoreValve® devices: results from the France 2 Registry. Catheter Cardiovasc Interv 2015;86:E158–66. [DOI] [PubMed] [Google Scholar]
- 42. Nadeem F, Tsushima T, Ladas TP, Thomas RB, Patel SM, Saric Pet al. . Impact of right ventricular pacing in patients who underwent implantation of permanent pacemaker after transcatheter aortic valve implantation. Am J Cardiol 2018;122:1712–7. [DOI] [PubMed] [Google Scholar]
- 43. Nazif TM, Dizon JM, Hahn RT, Xu K, Babaliaros V, Douglas PSet al. . Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: the PARTNER (Placement of AoRtic TraNscathetER Valves) trial and registry. JACC Cardiovasc Interv 2015;8:60–9. [DOI] [PubMed] [Google Scholar]
- 44. Nijenhuis VJ, Dijk VV, Chaldoupi SM, Balt JC, Berg JT.. Severe conduction defects requiring permanent pacemaker implantation in patients with a new-onset left bundle branch block after transcatheter aortic valve implantation. Europace 2017;19:1015–21. [DOI] [PubMed] [Google Scholar]
- 45. Pereira E, Ferreira N, Caeiro D, Primo J, Adão L, Oliveira Met al. . Transcatheter aortic valve implantation and requirements of pacing over time. Pacing Clin Electrophysiol 2013;36:559–69. [DOI] [PubMed] [Google Scholar]
- 46. Rogers T, Devraj M, Thomaides A, Steinvil A, Lipinski MJ, Buchanan KDet al. . Utility of invasive electrophysiology studies in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. Am J Cardiol 2018;121:1351–7. [DOI] [PubMed] [Google Scholar]
- 47. Schymik G, Tzamalis P, Bramlage P, Heimeshoff M, Würth A, Wondraschek Ret al. . Clinical impact of a new left bundle branch block following TAVI implantation: 1-year results of the TAVIK cohort. Clin Res Cardiol 2015;104:351–62. [DOI] [PubMed] [Google Scholar]
- 48. Urena M, Webb JG, Tamburino C, Muñoz-García AJ, Cheema A, Dager AEet al. . Permanent pacemaker implantation after transcatheter aortic valve implantation impact on late clinical outcomes and left ventricular function. Circulation 2014;129:1233–43. [DOI] [PubMed] [Google Scholar]
- 49. Walther T, Manoharan G, Linke A, Möllmann H, Holzhey D, Worthley SGet al. . Incidence of new-onset left bundle branch block and predictors of new permanent pacemaker following transcatheter aortic valve replacement with the PorticoTM valve. Eur J Cardiothorac Surg 2018;54:467–74. [DOI] [PubMed] [Google Scholar]
- 50. Sweeney MO, Hellkamp AS.. Heart failure during cardiac pacing. Circulation 2006;113:2082–8. [DOI] [PubMed] [Google Scholar]
- 51. Steinberg JS, Fischer A, Wang P, Schuger C, Daubert J, Mcnitt S, et al. ; MADIT II Investigators . The clinical implications of cumulative right ventricular pacing in the multicenter automatic defibrillator trial II. J Cardiovasc Electrophysiol 2005;16:359–65. [DOI] [PubMed] [Google Scholar]
- 52. Sharma AD, Rizo-Patron C, Hallstrom AP, O’Neill GP, Rothbart S, Martins JBet al. . Percent right ventricular pacing predicts outcomes in the DAVID trial. Hear Rhythm 2005;2:830–4. [DOI] [PubMed] [Google Scholar]
- 53. Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KLet al. . Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003;107:2932–7. [DOI] [PubMed] [Google Scholar]
- 54. Bruno F, D’Ascenzo F, Vaira MP, Elia E, Omedè P, Kodali Set al. . Predictors of pacemaker implantation after transcatheter aortic valve implantation according to kind of prosthesis and risk profile: a systematic review and contemporary meta-analysis. Eur Heart J - Qual Care Clin Outcomes 2021;7:143–53. [DOI] [PubMed] [Google Scholar]
- 55. Elzeneini M, Assaf Y, Aalaei-Andabili SH, Mahmoud A, Hamburger R, Goel Ret al. . Predictors of ventricular pacing burden after permanent pacemaker implantation following transcatheter aortic valve replacement. Clin Cardiol 2020;43:1334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yasser S, Kinjal B, Arnav K, Hassan L, Sanchit C, Cameron Iet al. . Systematic approach to high implantation of SAPIEN-3 valve achieves a lower rate of conduction abnormalities including pacemaker implantation. Circ Cardiovasc Interv 2021;14:e009407. [DOI] [PubMed] [Google Scholar]
- 57. Tang GHL, Zaid S, Michev I, Ahmad H, Kaple R, Undemir Cet al. . “Cusp-overlap” view simplifies fluoroscopy-guided implantation of self-expanding valve in transcatheter aortic valve replacement. JACC Cardiovasc Interv 2018;11:1663–5. [DOI] [PubMed] [Google Scholar]
- 58. Pisaniello AD, Makki HBE, Jahangeer S, Daniels MJ, Hasan R, Fraser DGW.. Low rates of permanent pacing are observed following self-expanding transcatheter aortic valve replacement using an annular plane projection for deployment. Circ Cardiovasc Interv 2021;14:e009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in Supplementary material online.