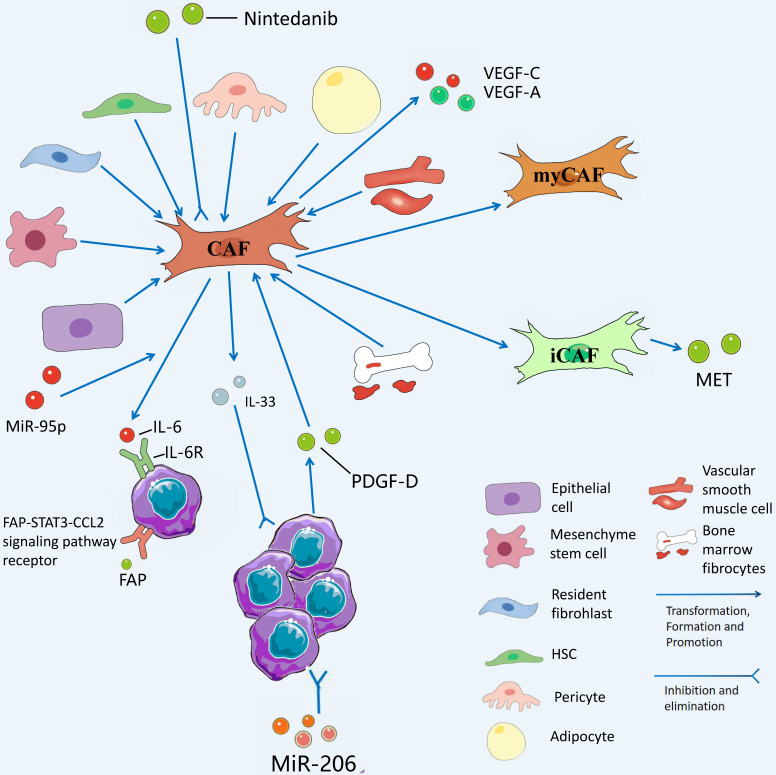
Figure 3.
CAFs has diverse sources and is differentiated from epithelial cells, mesenchyme stem cells, resident fibrohlast, HSCs, pericyte, adipocyte, vascular smooth muscle cells, and bone marrow fibrocytes. CAFs from hepatic stellate cells (HSCs) mediate the release of hepatocyte growth factor from inflammatory CAFs through direct interaction of the hsc-cafa-tumor pathway and promote the proliferation of ICC through tumor-expressed MET. VCAFs (vascular carcinoma-associated fibroblasts) secrete IL-6 (interleukin-6) to enhance the malignancy of ICC cells through the interaction of the IL-6/IL-6R axis with tumor cells, while exosomal miR-95p of ICC cells can induce IL-6 expression in vCAFs. High expression of miR-34c in tumor-derived exosomes can target and inhibit Wnt1, allowing it to activate the Wnt signaling pathway in CCA and slow the conversion of fibrocytes to CAFs. Nintedanib can treat refractory CCA by inhibiting the activation of CAFs.