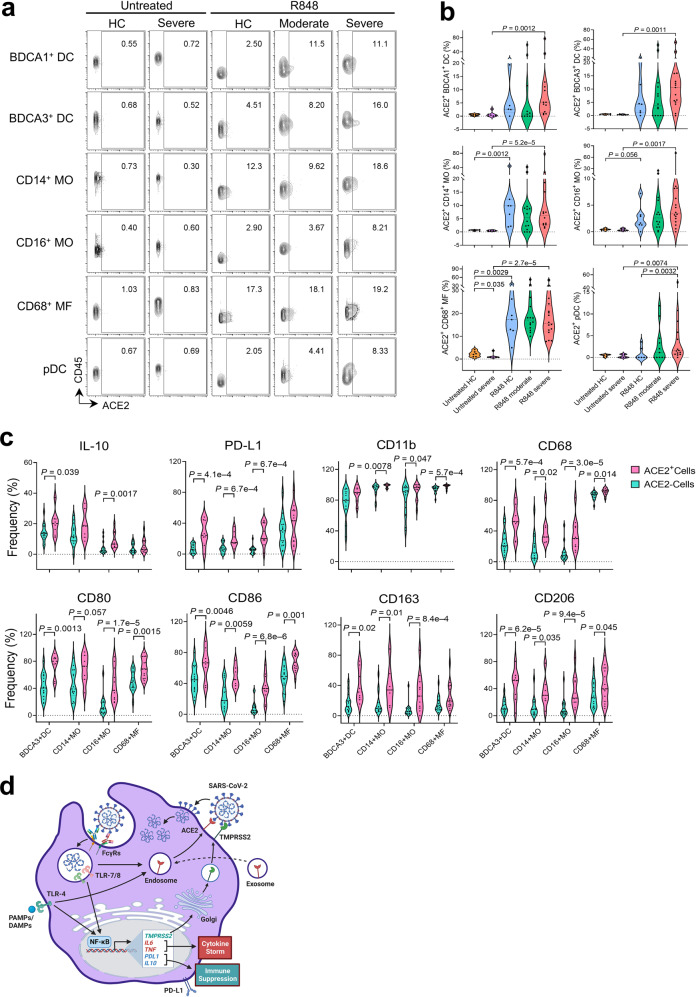
Fig. 6. ACE2 surface translocation upon ex vivo R848 stimulation is positively associated with hyperactivation responses and PD-L1 expression in blood myeloid cells from COVID-19 patients.
a Mass cytometry analysis of cell surface ACE2 expression in blood myeloid populations from untreated samples (n = 7 HC; n = 7 severe COVID-19) and R848-treated samples (n = 7 HC; n = 15 moderate COVID-19; and n = 16 severe COVID-19). b Violin plots of frequencies of the myeloid compartment expressing surface ACE2 as shown in a. c Frequencies of ACE2+ and ACE2– cells (based on ACE2 surface expression) within the myeloid compartment expressing the indicated markers in cells from severe COVID-19 patients. d Graphic summary of SARS-CoV-2 infection in monocytes co-expressing surface ACE2 and TMPRSS2 upon TLR4/7/8 activation. ACE2 is taken up by monocytes from ACE2-containing exosomes and stored in the early endosome at steady state. TLR7/8 activation triggered by endocytosis of SARS-CoV-2 or TLR4 activation triggered by viral proteins or host-derived danger signals released during infection stimulates downstream TLR signaling pathways to enhance gene expression of TMPRSS2, proinflammatory cytokines, IL-10, and PD-L1, which drive the cytokine storm and promote immune suppression. TLR4/7/8 activation also induces ACE2 translocation through endosomal trafficking to the cell membrane. Translocated ACE2 and newly synthesized TMPRSS2 at the cell surface facilitate SARS-CoV-2 viral entry and active replication in monocytes. Image generated with BioRender.