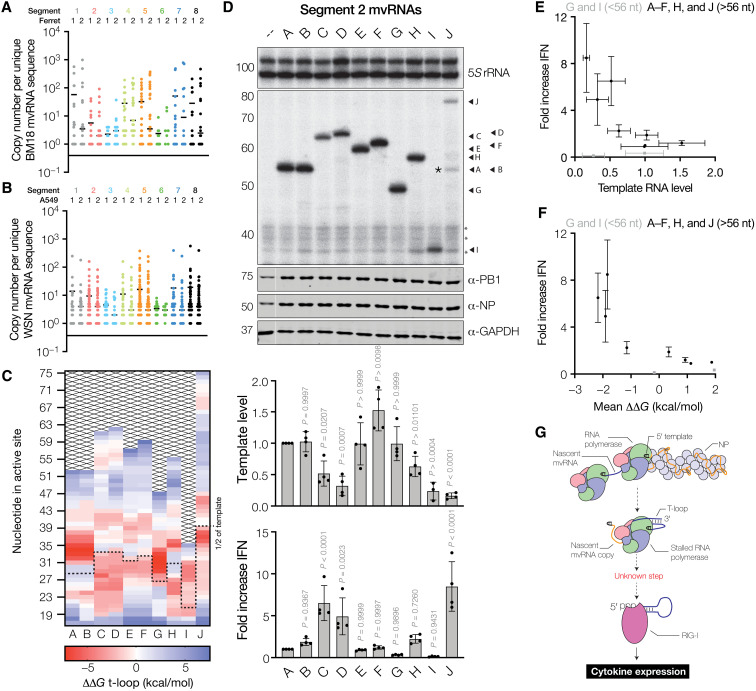
Fig. 5. Reduced mvRNA replication in viral infections is correlated with IFN-β promoter activation.
(A) Number of mvRNA copies per unique mvRNA sequence detected in ferret lungs infected with BM18 or (B) A549 cells infected with WSN. In each graph, two biological repeats are shown. (C) ΔΔG heatmap of negative-sense template of WSN segment 2–derived mvRNAs. Half of the mvRNA template is indicated with a dotted line. (D) Replication of segment 2–derived mvRNAs identified by NGS in HEK293T cells by the WSN RNA polymerase. RNA levels were analyzed by primer extension. The ability of mvRNA replication to induce IFN-β promoter activity was analyzed using a luciferase reporter assay. Asterisk (*) indicates nonspecific radioactive signal. Data from four biological repeats are shown. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (E) IFN-β induction is negatively correlated with template replication level. PB1-mvRNA G and I were excluded from the fit to the exponential decay, because they are shorter than the IFN-β promoter induction cut-off of 56 nt. (F) IFN-β induction is negatively correlated with ΔΔG of first half of template mvRNA. (G) Schematic of aberrant RNA synthesis by the IAV RNA polymerase. T-loops present in some mvRNAs lead to reduced RNA polymerase processivity. This may induce template release and/or binding of the mvRNA template to RIG-I. Host factor Acidic Nuclear Phosphoprotein 32 Family Member A (ANP32A), which plays a key role during cRNA and vRNA synthesis, is not shown for clarity (16, 43).