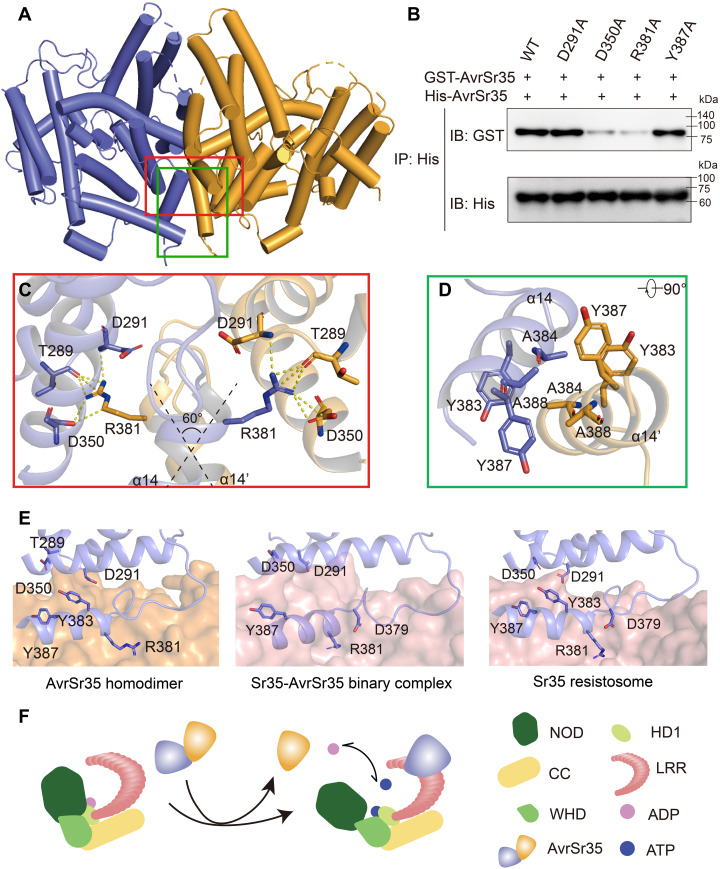
Fig. 4. Mechanism of Sr35 activation by AvrSr35.
(A) The crystal structure of the AvrSr35 dimer is shown in ribbon representation with subunits A and B colored slate and orange, respectively. (B) AvrSr35 mutants around interaction interface shown in (C) and (D) diminish the homodimerization interaction. IP: immunoprecipitation; IB: immunoblotting. (C) Detailed view of hydrophilic interactions between two copies of AvrSr35. (D) Bottom view of hydrophobic interactions between two copies of AvrSr35. (E) Comparison of the interface between different states of Sr35 activated by AvrSr35. Left: AvrSr35 dimerization interface that one protomer represented in orange surface and the other protomer shown in slate cartoon. Middle: Interaction surface of the intermediate state of AvrSr35 and Sr35 complex in the absence of ATP. Sr35 LRR is shown as salmon surface, and AvrSr35 is shown as slate cartoon. Right: Interaction surface of the activated state of Sr35 resistosome. Sr35LRR is shown as salmon surface, and AvrSr35 is shown as slate cartoon. Interacting residues are shown as sticks and labeled accordingly. (F) Diagram depicting the binding of AvrSr35 with Sr35.