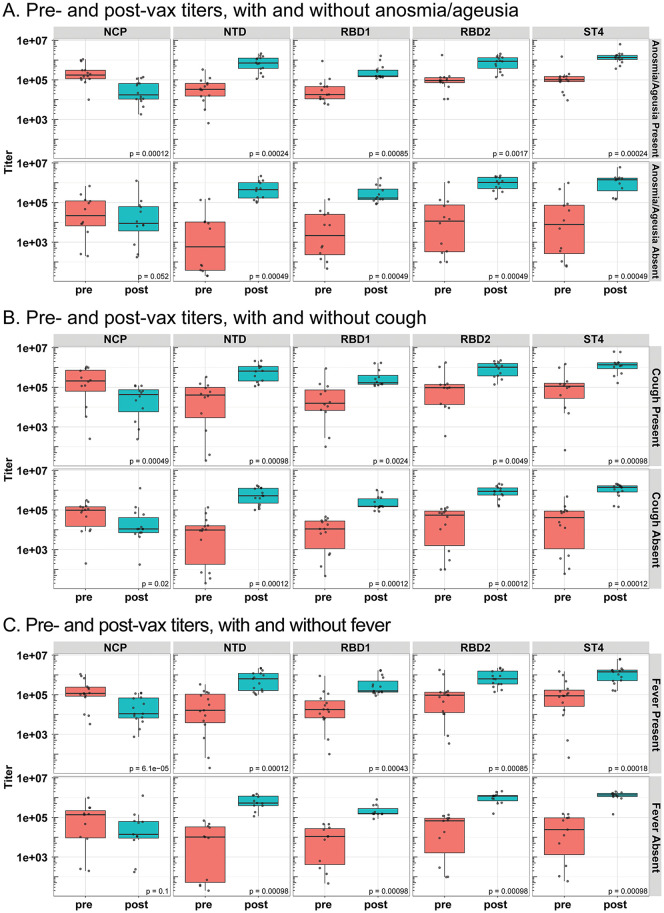
Fig 4. Pre- & post-vaccinated participant SARS-CoV-2 antibody titer comparisons in presence and absence of anosmia/ageusia, cough, and fever.
Spike protein antibody titers were analyzed in convalescent plasma samples from pre-vaccinated participants that returned for the post-vaccinated visit (n = 27, red labeling), and participants from the post-vaccinated visit (n = 27, blue labeling). Pre- and post-vaccinated participant titer comparison for NCP, NTD, RBD1, RBD2, and ST4 within participants that reported a presence or absence of anosmia/ageusia (A), cough (B), and fever (C) in symptom questionnaires. Boxes and horizontal bars denote the interquartile range (IQR). The whiskers are equal to the maximum and minimum titer values below or above the median at 1.5 times the IQR. Statistical significance between groups was determined by non-parametric t-test. The differences were considered statistically significant when p<0.05. Pre/pre-vax = pre-vaccinated, post/post-vax = post vaccination.