Figure 1.
Serological responses to 3rd and 4th dose vaccination.
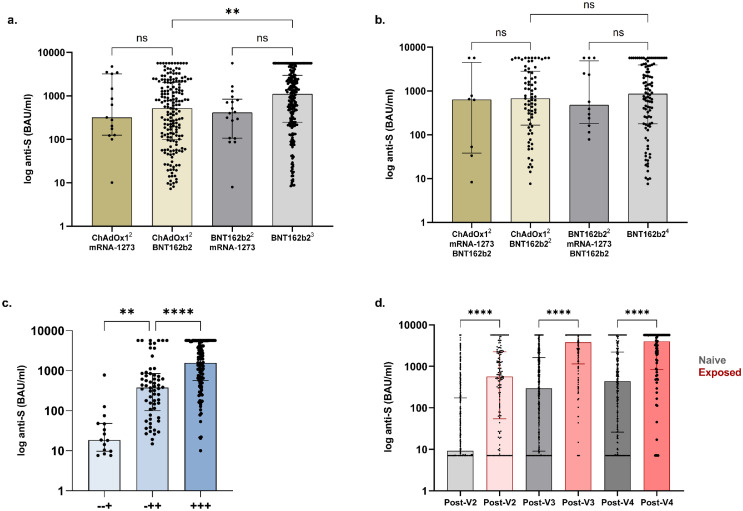
(A) Anti-S concentrations post-V3 in infection-naïve patients receiving ChAdOx12-mRNA1273, ChAdOx12-BNT162b2, BNT162b22-mRNA1273 and BNT162b23, were 319 (125-3213), 518 (98-2049), 412 (106-841) and 1110 (246-2969) BAU/ml respectively. Significantly higher concentrations were seen with BNT162b2 as V3 following priming with BNT162b2 compared with ChAdOx1, p=0.0011 (B) Anti-S concentrations post-V4 in infection-naïve patients receiving ChAdOx12-mRNA1273-BNT162b2, ChAdOx12-BNT162b22, BNT162b22-mRNA1273-BNT162b2 and BNT162b24, were no different between groups. (C) Anti-S concentrations post-V4 by dose of vaccine seroconverted. +++, -++, and -+ denote seroconversion post V2, V3 and V4 respectively. The median anti-S in infection-naïve patients post-V4 in those seroconverting post V2-, V3- or V4- were 1561 (567-5211), (101-851) and 19 (9.7-48) BAU/ml respectively. (D) Anti-S concentrations post 2nd-, 3rd- and 4th vaccinations by infection exposure Anti-S concentrations were greater in patients with prior infection (568 (54-2237) post-V2, 3791 (1142-5680) post-V3 and 3993 (835-5680) BAU/ml post-V4) compared with infection-naïve patients (9.2 (7.1-173) post-V2, 295 (9.1-1611) post-V3 or 437 (26-2211) BAU/ml post-V4). There was no difference between post-V2 concentrations in patients with prior infection compared with infection naïve individuals post-V3, p=0.06 or post-V4, p=0.99.