Abstract
Older kidney transplant recipients demonstrate increased rates of infection, and lower rates of rejection, compared with younger kidney transplant recipients. However, the mechanism behind this observation remains unknown. To develop a multifaceted view of age-associated immune dysfunction, we determined the function and phenotype of T cells predisposing to vulnerability to infection on a molecular level.
Overlapping peptide pools representing the dominant CMV antigens were used to stimulate PBMC collected from 51 kidney transplant recipients, using cytokine secretion to determine specificity and intensity of response. Staphylococcal endotoxin B (SEB) was analyzed in parallel. To define immune cell subsets, we used single cell RNA sequencing (scRNAseq) to evaluate cellular surface markers and gene expression.
We found increased frequency of SEB- and CMV-specific T cells was associated with freedom from infection, especially in older patients. Spatialized t-SNE analysis revealed decreased frequency of naïve T cells, increased frequency of TEMRA cells, and decreased frequency of IFNγ secreting T cells in patients with infection. Application of scRNAseq analysis revealed increased frequency of terminally differentiated T cells expressing NK-associated receptors and inhibitory markers.
These findings offer unique insight into the mechanism behind vulnerability to infection in the kidney transplant recipient, revealing a specific T cell subtype of impaired antigen response and terminal effector phenotype as markers of T cell senescence.
Keywords: Transplantation, Aging, T cell, Senescence, Exhaustion, Inflammation, IFNγ
1. Introduction
Kidney transplant recipients prescribed lifelong immunosuppression to prevent rejection are at risk for infection, especially older patients who are at increased risk for infectious complications and death compared to younger transplant recipients [1–4]. The number of older kidney transplant candidates and recipients continues to grow, driven in large part by the aging population and increased incidence of kidney disease in older patients [5–6]. In 2019, patients older than age 65 made up 24% of patients on the waiting list and 22% of the adult kidney transplant recipients [7].
Studies of older individuals have identified many age-associated changes in T cell function that are speculated to account for the increased vulnerability to infection and poor response to vaccination seen in older individuals [8–10]. After thymic involution, older individuals demonstrate a decrease in the frequency of naïve T cells and an increase in frequency of terminally differentiated effector memory cells expressing CD45RA, or TEMRA cells, which demonstrate decreased proliferation capacity as well as increased expression of pro-inflammatory cytokines [9,11]. A related age-associated change is T cell senescence, defined as the loss of ability to proliferate after antigen stimulation, which is associated with a reduction in telomere length and diminished expression of CD28 [11–12]. T cell exhaustion is a distinct aspect of age-associated dysfunction, characterized by the expression of inhibitory receptors such as PD-1, leading to requirement of a higher threshold for activation and reduced functionality after activation, and is observed in the context of chronic viral infection with repeat T cell receptor engagement [10,13–14]. Anergy is another feature of T cell dysfunction found in the setting of hypostimulation and engagement of T regulatory cells, and is associated with impaired cytokine secretion. CMV infection is known contributor to immune aging through a process known as immune inflation, where the expansion and maintenance of CMV-specific CD8 + T cells increases through an individual’s lifespan, increasing the frequency of senescent T cells and concurrently reducing the available pool of naïve T cells [15–16].
Our previous investigations demonstrated that senescent and exhausted T cells are found in increased frequency in kidney transplant recipients who develop infection after transplantation [17]. Another important contributor to T cell dysfunction is history of CMV infection, which can drive immune senescence and exhaustion [18]. The CMV-specific immune response can predict control of CMV [19], but whether it can also predict vulnerability to infections other than CMV has not been explored.
Another ongoing question in transplant immunology is whether the functional impairment associated with the aging-associated immune senescent phenotype is associated with infection in kidney transplant recipients receiving immunosuppression, and which subtypes of impaired T cells are most important for immune control. We sought to determine whether functional assessment of T cell response to infection-related antigens would be associated with infection after kidney transplantation by evaluating response to Staphylococcal and CMV antigen in a parallel with evaluation of immune phenotype.
2. Methods
2.1. Patient cohort
Patients were enrolled in an observational clinical study approved by the UCLA IRB as previously described [20]. Older kidney transplant recipients (≥age 60) with blood samples available at 3 months post transplant were cohort matched with younger patients (ages 30–59) by transplant type (living versus deceased) and use of induction immunosuppression (antithymocyte globulin versus basiliximab). PBMC were isolated and stored prior to analysis. Maintenance immunosuppression at our center includes tacrolimus, mycophenolate mofetil, and prednisone, in conjunction with antibiotic prophylaxis with valganciclovir for three months for seropositive recipients and six months for high-risk donor positive recipient negative patients per UCLA clinical protocol [20].
Chart review was performed to identify episodes of infection using Infectious Diseases Society of America definitions for bacterial, fungal, and viral infections, including both CMV DNAemia and CMV disease. Patients were described in the “infection” group if they developed CMV or non-CMV infection in the first year post transplantation, but “no infection” if they did not develop infection during this first year. For the purposes of this analysis, urinary tract infection was not included as a validated infection unless accompanied by pyelonephritis or bacteremia given the difficulty in interpreting positive urine cultures in the absence of clear symptoms.
2.2. Flow cytometry
PBMCs were thawed overnight and then incubated for 8 h with monoclonal anti-CD28 (L293) and anti-CD49a (L25) antibodies (CD28/CD49d; BD Biosciences), Brefeldin A (Golgi plug; BD Biosciences) and one of the following stimuli: (i) no stimulation, (ii) Staphylococcal enterotoxin B (SEB, 3 ug/ml), or (iii) overlapping 15 amino acid peptide pools representing CMV virus proteins from the 9 most immunodominant antigens (JPT Peptide Technologies) at a concentration of 5 lg/mL, namely UL55, UL83 (pp65), UL99, UL36, UL48_sub1, UL48_sub2, UL122 (IE-1), UL123 (IE-2), US32 [21]. Cells were stained for surface markers with fluorochromeconjugated antibodies against CD3 (PCP-Cy5, OKT3 clone), CD4 (PE-CF 594, RPA-T4 clone), CD8 (APC-H7-CD8, SK1 clone), CCR7 PE-Cy7, G043H7 clone), IFNγ (FITC, B27 clone), TNFα (A700, MAb11 clone), IL-2 (PE, MQ1–17H12 clone), (BD Biosciences or Biolegend), fixed, and then permeabilized for intracellular cytokine staining followed by analysis by the BD LSRFortessa (BD Biosciences) using FCS Express software (DeNovo Software), as previously reported [22]. For CMV analysis, only patients at risk for CMV infection as measured by either donor or recipient seropositivity were included in the analysis.
2.3. Network-based visualization of flow cytometry data
Raw FCS files were imported into R and analyzed as described below, using R base packages and ConsensusClusterPlus, flowCore and flowWorkspace. A spillover matrix, defined using single-stain FCS files, was used to compensate for spillover between channels. Dead cells and doublets were removed, and raw MFI values were arcsinh transformed with a cofactor parameter of 150. Clusters of cytokine-positive CD3+ CD4+ T cell and CD3+ CD8+ T cell subsets, expressing one or more of IFNy, IL-2 or TNFα, were identified in an unsupervised manner using the FlowSOM algorithm, which initially defined 100 clusters using a Self-Organizing Map (SOM). These clusters were combined into 40 meta-clusters by hierarchical clustering.
For visualization, each subset was subsampled to 10,000 cells with equal representation per patient. To ensure the subsampled cells per patient reflected the cluster distribution of their complete dataset, we generated 100 random subsamples per patient and chose the set which most closely matched the cluster distribution of the complete dataset. Normalized expression of all surface markers for the subsampled cells was reduced to two dimensions, using t-distributed stochastic neighbor embedding (t-SNE), with a perplexity value of 75. A graph was generated with cells as nodes, distributed in two dimensions using the described t-SNE. Pairwise correlation of marker expression between cells were utilized as edge weights, and edge weights were used to visually spatialize the cells in Gephi using the ForceAtlas2 algorithm. Cell cluster membership and marker expression were overlayed on the graph-based visualizations in R.
2.4. Single-cell CITE-Seq analysis of CD8+ T cells
A total of six CMV seropositive kidney transplant recipients were selected for further analysis from the previously analyzed cohort described above, 3 with history of CMV DNAemia and 3 without, matched on sex and induction immunosuppression. CD8+ T cells were negatively selected from thawed PBMCs using MACS magnetic beads, dead cells were similarly removed. Cells were stained for CD4, CD8, CCR7, CD45A, CD57, CD28, and PD-1 using TotalSeq B reagents per manufacturer recommendations. Single cell libraries of a total of 14,393 stained, cells from 6 patient samples were prepared using Chromium Single Cell 30′ kits (10X Genomics) before pooling and sequencing on the NextSeq instrument. Raw fastq files for antibody (Feature Barcode) and gene expression (transcript) libraries were processed using Cell Ranger 5.0.1 to generate antibody, gene expression count matrices. Data from all patients was integrated using Cell Ranger aggr. Data was QC’ed and analyzed in R, primarily using Seurat. Cells with >4000 or <200 unique features (genes) or mitochondrial counts >15% were removed. Cells identified as B, CD4+ T or NK cells based on gene expression profile were removed prior to downstream analyses. Using the top 2000 most variable genes and 30 principal components, 24 clusters of CD8+ T cells were identified. Genes upregulated or downregulated per cluster were identified using the FindAllMarkers function. Complete gene expression profiles of all cells were reduced to 2 dimensions using t-SNE analysis for visualization purposes.
2.5. Statistical analysis
Statistical analysis for data reported in Tables was performed using Jmp Pro 13 using Kruskal-Wallis tests on all numeric variables given the observed nonparametric distribution. Additional analysis was performed using linear regression for data described in Figures (R Core Team 2021). A p-value of 0.05 or less was considered statistically significant.
3. Results
3.1. Demographics of patients analyzed
We evaluated 51 kidney transplant recipients ages 34–80 years old with blood samples available for testing at 3 months post-transplant (Table 1). Of the patients who experienced infection, 50% were related to CMV, and 50% were non-CMV infections including 5 bacterial infections (2 systemic, 1 respiratory, 1 gastrointestinal, and 1 pyelonephritis), 2 non-CMV viral infections (1-gastrointestinal and 1 respiratory), and 1 fungal infection (oral cavity), as described previously [23]. A trend was observed towards increased age in patients experiencing infection which did not reach statistical significance (p = 0.240). Patients with infection compared with no infection were similar in other attributes including induction immunosuppression and deceased versus living donor status, although patients with infection were more likely to be male. As described previously, infection types included bacterial pneumonia, bacteremia, pyelonephritis, cellulitis, oral candidiasis, norovirus-associated diarrhea, viral upper respiratory infection, and CMV DNAemia [23].
Table 1.
Demographic and clinical characteristics of patients with and without infection after kidney transplantation. Nonparametric testing for numeric variables and Pearson test for categorical variables.
| Total cohort (n = 51) | Infection (n = 16) | No Infection (n = 35) | p-value | |
|---|---|---|---|---|
|
| ||||
| Age, years (median, range) | 47 (34–80) | 54(36–77) | 47 (34–80) | 0.715 |
| Donor type (% Deceased donor) | 43.1% | 56.3% | 37.1% | 0.201 |
| Induction type (% ATG) | 27.5% | 31.3% | 25.7% | 0.681 |
| Sex (% Male) | 66.7% | 87.5% | 57.4% | 0.033 |
| Hispanic ethnicity | 35.3% | 18.8% | 42.9% | 0.095 |
| Race (% White) | 64.7% | 62.5% | 65.7% | 0.824 |
| CMV high risk (D+/R−) | 15.7% | 25.0% | 11.4% | 0.240 |
| CMV intermediate risk (R+) | 72.5% | 68.8% | 74.3% | 0.651 |
3.2. Impaired SEB antigen response and association with infection
Analysis of response to SEB as a marker of general T cell function in terms of single cytokine production demonstrated increased frequency of cytokine secretion in IFNγ+ CD8+, IL2+ CD4+, and TNFa+/IL-2+ CD4+ T cells in patients without infection in the first year after transplantation (Fig. 1, Supplemental Table 1). This difference was seen for single cytokine secretion including IFNγ+ CD8+ T cells, with 0.4% (IQR 0.2%–0.6%) for those without infection compared with 0.2% (IQR 0.1%–0.4%) for those with infection (p = 0.014). A similar trend towards significance was seen for IL-2+ CD8+ T cells, with 0.4% (IQR 0.2%–0.9%) frequency for those without infection compared with 0.2% (IQR 0.1%–0.6%) for those with infection (p = 0.086) (Fig. 1A). A significant difference was also seen between IL-2+ CD4+ T cells, with 0.6% (IQR 0.4%–0.8%) for those without infection and 0.3% (IQR 0.1%–0.4%) for those with infection (p = 0.008) (Fig. 1B). Interestingly, when patients were divided by age, IL-2+ CD4+ T cell expression was still significantly greater without infection in the ≥60 year old patients (p = 0.014) but there was no significant difference in the younger < 60 year old cohort (p = 0.243) (Fig. 1A).
Fig. 1.
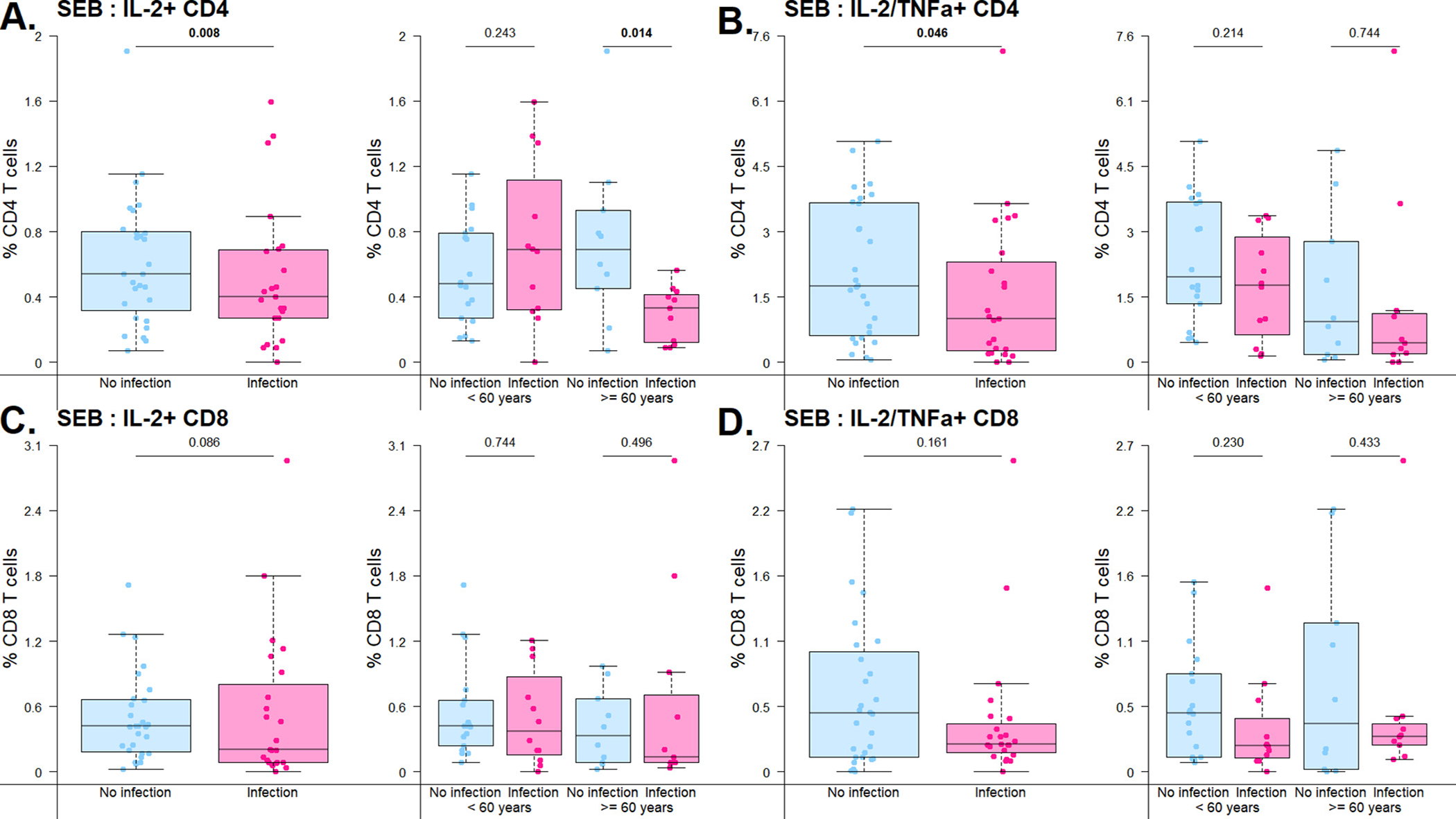
Frequency of cytokine-secreting cells detected after stimulation with SEB antigen. Bar and whiskers graph demonstrates median and IQR for patients with or without infection in the first year after kidney transplantation. Top panel represents CD4+ T cells and bottom panel represents CD8+ T cells. A) CD4+ T cell single cytokine secretion after stimulation with SEB, by overall cohort and by age group (<60 v 60 years old). B) CD4+ T cell double-cytokine secretion after stimulation with SEB, by overall cohort and by age group (<60 v ≥60 years old). C) CD8+ T cell single secretion after stimulation with SEB, by overall cohort and by age group (<60 v ≥60 years old). D) CD8+ T cell double-cytokine secretion after stimulation with SEB, by overall cohort and by age group (<60 v ≥60 years old). Analysis by linear regressions of subset percentage on infection status.
Similarly, analysis of double cytokine-secreting T cells revealed increased frequency of cells responding to SEB stimulation for TNFα+/IL-2+ CD4+ T cells, with a frequency of 1.8% (IQR 0.6%–3.7%) in patients without infection and 0.8% (IQR 0.2%–2.2%) in patients with infection (p = 0.046) (Fig. 1C). Analysis by age cohort also demonstrated that this difference is primarily driven by the older patient group, with increased frequency of double-cytokine secretion in those protected from infection (p = 0.744). A similar trend was observed for TNFα+/IL-2+ CD8+ T cells, with a frequency of 0.4% (IQR 0.1%–0.9%) in patients without infection and 0.2% (IQR 0.1%–0.3%) in patients with infection (Fig. 1D). Evaluation of triple cytokine-secreting T cells for CD4+ T cells, expressed as percentage of double cytokine secreting cells, showed a similar trend towards increased frequency in patients without infection (41.7%, IQR 24.1%–55.2%) as compared with those with infection (24.6%, IQR 8.0%–49.0%), although this did not reach statistical significance (p = 0.098). When analyzed by age group, triple cytokine secretion demonstrated a trend towards increased frequency in those protected from infection in both the older (infection, 0.0%, IQR 0.0%–1.1% and no infection, 35.1%, IQR 22.0%–61.3%, p = 0.027) and younger (infection, 10.6%, IQR 0.0%–42.9% and no infection, 24.2%, IQR 12.3%–38.2%, p = 0.873) age groups. Analysis by maturation subtype of double cytokine secreting cells did not reveal any significant differences between patients with and without infection (Supplemental Table 1).
3.3. Impaired CMV antigen response and association with infection
Given the importance of control of CMV for transplant recipients, and the known association between CMV and T cell senescence, we used immune response to the clinically relevant antigen CMV in order to further evaluate T cell function in transplant recipients. In patients with and without infection after transplantation, we analyzed response to CMV antigen for patients at risk for CMV infection, who were either donor or recipient seropositive for CMV. Analogous to the results for SEB response, we noted increased frequency of single cytokine secreting T cells after CMV antigen stimulation in patients without infection (Supplemental Table 2). This difference was most notable in CD4+ T cells, with a frequency of 0.3% (IQR 0.1%–0.8%) for patients without infection compared with 0.1% (IQR 0.1%–0.3%) for patients with infection for both TNFα+ CD4+ (p = 0.035), and 0.3% (IQR 0.1%–0.5%) for patients without infection compared with 0.1% (IQR 0.0%–0.3%) for IL-2+ CD4+ single cytokine secreting T cells (p = 0.026) (Fig. 2A). These differences were again most striking in the older patient cohort, with increased frequency of IL-2+ CD4+ T cells in those protected from infection the older patient cohort (p = 0.019), while for the younger patient cohort, the frequency of IL-2-secreting CD4+ T cells was similar in those with and without infection (p = 0.172).
Fig. 2.
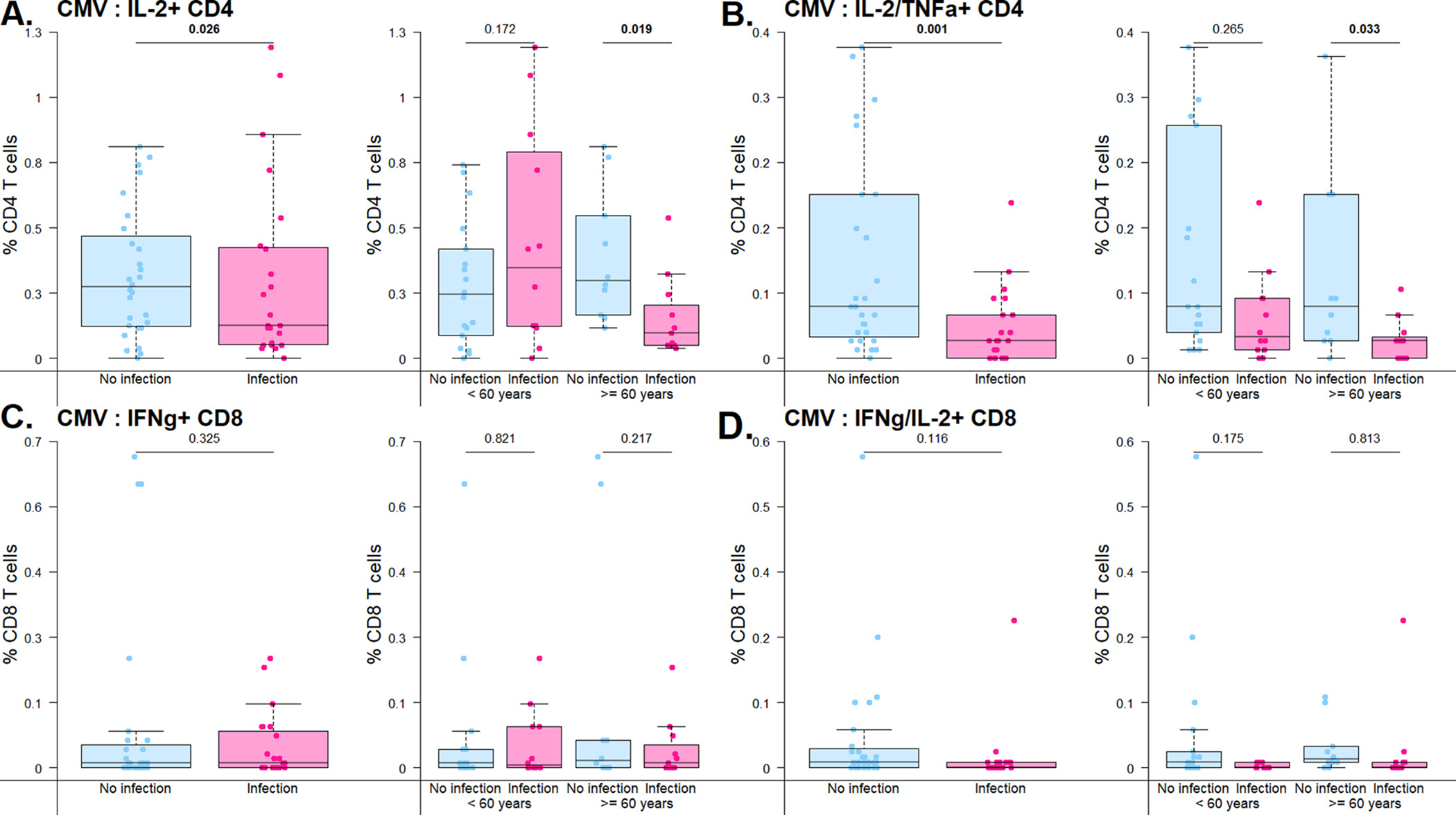
Frequency of cytokine-secreting cells detected after stimulation with CMV antigens. Bar and whiskers graph demonstrates median and IQR for patients with or without infection in the first year after kidney transplantation. Top panel represents CD4+ T cells and bottom panel represents CD8+ T cells. A) CD4+ T cell single cytokine secretion after stimulation with CMV peptides, by overall cohort and by age group (<60 v ....60 years old). B) CD4+ T cell double-cytokine secretion after stimulation with CMV peptides, by overall cohort and by age group (<60 v ≥60 years old). C) CD8+ T cell single secretion after stimulation with CMV peptides, by overall cohort and by age group (<60 v ≥60 years old). D) CD8+ T cell double-cytokine secretion after stimulation with CMV peptides, by overall cohort and by age group (<60 v ≥60 years old). Analysis by linear regressions of subset percentage on infection status.
This observation was also seen when double-cytokine secreting T cells were evaluated, with significant differences in frequency of TNFα+/IL-2+ CD4+ T cells for patients with versus without infection after kidney transplantation (p = 0.001) (Fig. 2B). This difference was also seen for IFNγ+/IL-2+ CD4+ T cells (p = 0.003) and IFNc+/TNFa+ CD4+ T cells, with a frequency of 0.1% (IQR 0.0%–0.3%) for patients without infection compared with 0.0% (IQR 0.0%–0.1%) for patients with infection (p = 0.006) (Supplemental Table 2). These differences were impacted by patient age, with increased frequency of IL-2+/TNFα+ CD4+ T cells in those protected from infection the older patient cohort (p = 0.033), a difference which was less striking when analysis was restricted to the younger patient cohort. A similar pattern was seen for CD8+ T cells expressing IFNγ or IFNγ+/IL-2+ although this observation did not reach statistical significance (Fig. 2C and 2D).
CD4+ triple cytokine secreting cells were similarly increased in frequency for patients without infection (26.9%) compared with those with infection (0.0%) after kidney transplantation (p = 0.015). Analysis of triple cytokine by age cohort also demonstrated differences in older (p < 0.027) compared with younger patients (p = 0.873). Interestingly, evaluation of maturation subtype for double cytokine secreting cells demonstrated the most prominent differences were in naïve subtype cells for both CD8+ and CD4+ T cells (p = 0.021 and 0.012, respectively). This difference was also seen in terminally differentiated effector memory (TEMRA) CD8+ T cells, with a frequency of 33.3% in patients without infection compared with 0.0% in patients with infection (p = 0.013).
3.4. Global analysis of CMV-specific immune response
To further characterize phenotypic differences in T cells responding to antigenic stimulation, CD4+ and CD8 + T cells producing at least one cytokine in response to CMV stimulation were divided into clusters in an unsupervised manner based on expression of surface markers corresponding to maturation subtype, ranging from naïve cells shown in green to TEMRA cells shown in dark red (Fig. 3A). Unsupervised clusters were visualized by t-SNE with cytokine expression overlayed, demonstrating associations between cytokine expression and memory subtypes for both CD8+ and CD4+ T cells (Fig. 3B). When clusters were categorized by single or double cytokine expression, the frequency of IL-2 or IFNγ expression in the effector memory maturation subtype differed in patients without infection in the first-year post-transplant (p = 0.045) (Fig. 3C). These differences demonstrated similar patterns for both older and younger patients for some subtypes (CD8+ IFNγ+ and IFNγ/IL-2+). However, for CD4+ IL-2+ and IL-2+/TNFα, increased frequency of effector memory was more strongly associated with protection from infection in the older patient cohort compared with younger patients (IL-2+, p = 0.027, IL-2+/TNFα+, p = 0.116). In contrast, frequency of TEMRA CD4+ IL-2+ cells was greater in those with increased vulnerability to infection in the younger patient group, although this did not reach significance (p = 0.296).
Fig. 3.
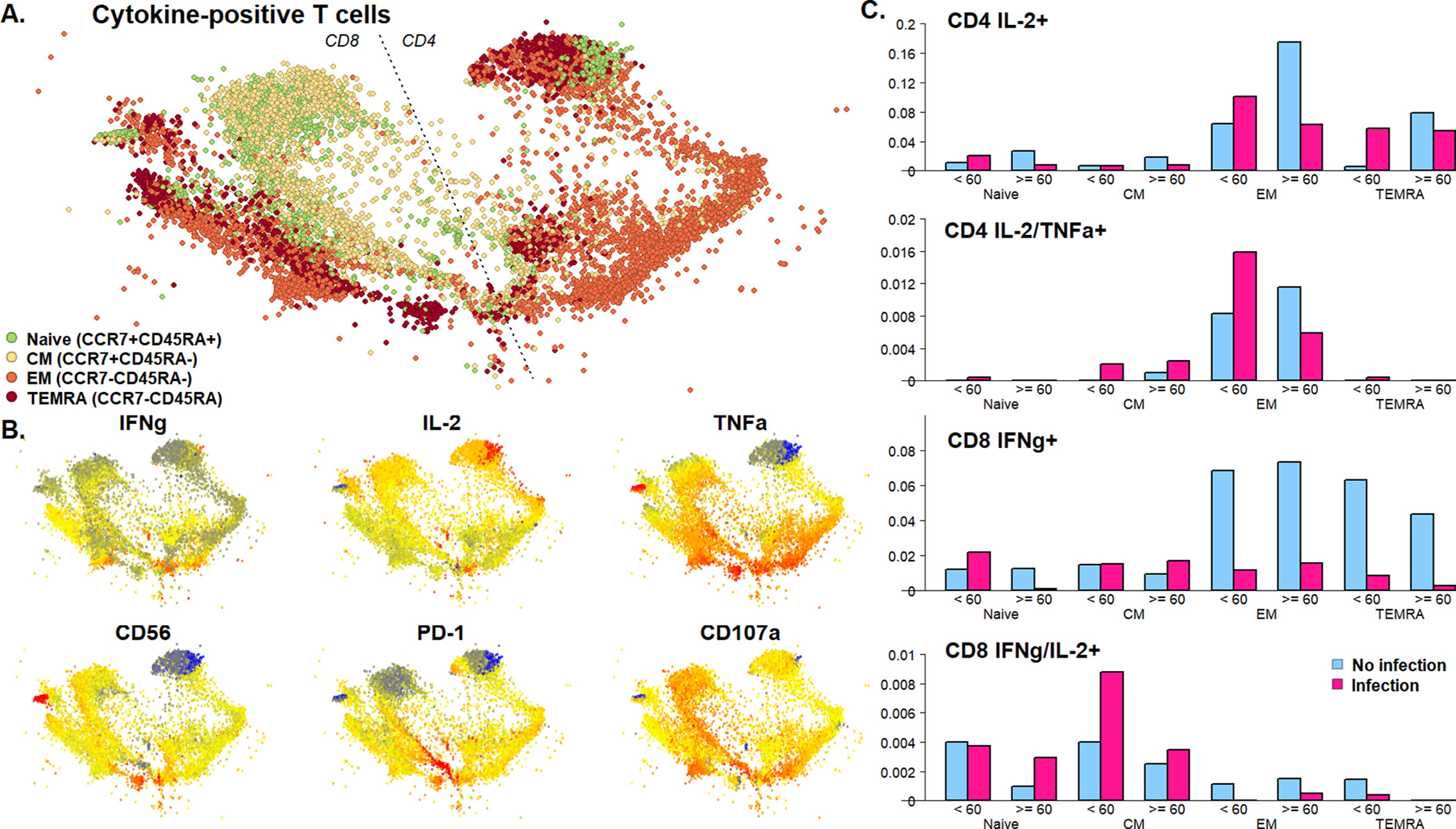
A) Spatialized t-SNE visualization of cytokine producing clusters of T cells. Total live cells were gated on CD4+ or CD8+ T cells producing at least one of IFNy, IL-2 and TNFa. Subsetted cells were assigned to 40 unsupervised clusters using the FlowSOM algorithm. For visualization, we further subsetted to 200 cells per sample. Expression of all markers was reduced to two dimensions by t-SNE followed by networks spatialization of cells using the ForceAtlas2 algorithm, with pairwise correlation of cells defining connectivity. Visualized cells are colored by cluster membership as shown. B) Normalized expression of surface markers and cytokines on spatialized t-SNE visualization. Raw MFI were arcsinh transformed with a cofactor of 150. Key indicates MFI low (blue) to high (red) intensity. C) Summed proportion of T cell maturation subtypes in single- and double cytokine-positive cells by infection status and age group.
3.5. Single cell analysis of gene transcription in patients with and without infection
Single cell RNA sequencing (scRNAseq) analysis of CD8+ T cells from patients with and without reactivation of CMV demonstrated a striking differentiation between by t-SNE analysis (Fig. 4A). Patients without infection had an abundance of CCR7+ naïve and central memory cells expressing AIF1, LEF1, SELL (CD62 ligand) and CCR7 (Fig. 4B–D, clusters 7 and 9). In addition, patients without infection displayed increased frequency of GZMK+ effector cells expressing markers of activation such as ITGB1 and LMNA (clusters 5 and 10). In contrast, patients with infection had increased abundance of cells with a more terminally differentiated phenotype (Fig. 4B–D, clusters 3 and 13). These terminally differentiated were distinguished by increased expression of genes involved in cytotoxic function (GZMB and GNLY), effector function (ZEB2), NK-associated receptors (KLRD1 and NKG7), and inhibitory markers associated with T cell dysfunction and/or senescence (LAIR2 and HOPX). Interestingly, effector marker CD107a was detected both in patients with and without infection, while IL-2 gene expression was rare. Expression of TNFα was associated with protection from infection, with four out of five clusters in which >20% of cells were TNFα+=+ were identified in the patients without infection, while IFNγ+ clusters were identified frequently in patients both with and without infection.
Fig. 4.

A) t-SNE visualization of CD8 T cells using single-cell transcriptomics. Graph-based clustering was utilized to define the number of clusters and cluster membership in an unsupervised manor. Cells from subjects with no infection (blue) and with infection (pink) are indicated, with darkness of the blue and pink respectively representing the 24 identified clusters. B) Number of cells in each cluster for controllers and non-controllers. Upregulated transcripts per cluster. Proportion of cells per cluster expressing a given transcript (colored blue/pink) and proportion of cells outside each cluster expressing a given transcripts (colored grey) are indicated. Clusters with increased abundance in controllers (blue) and non-controllers (pink) respectively are shown. C) Normalized expression of key transcripts distinguishing clusters of CD8 T cells. Normalized expression of transcripts projected onto the previously described t-SNE visualization of CD8 T cells, ranging from high expression (dark yellow) to low (light yellow) or no expression (grey).
4. Discussion
This multilevel analysis represents an extensive characterization of the T cell phenotypes associated with the clinically relevant outcome of infection associated with the biologically older patient. We found a strong association between antigen response as measured by cytokine release after stimulation with both SEB and CMV and freedom from infection. Clustering analysis demonstrated that the avoidance of infection is associated with increased frequency of CD4+ and CD8+ effector memory T cells producing one or multiple cytokines, with most striking association seen with double cytokine secretion in older patients. All patients with infection had increased frequency of CD8+ IFNγ+ TEMRA T cells regardless of age, while CD4+ IL-2+ TEMRA T cells had an age-dependent role, with vulnerability to infection associated with increased frequency in patients under 60 years old. We speculate that this difference may result from a stronger association with CD4+ T cells in preventing recurrent infection, especially CMV infection and/or reactivation, while CD8+ T cells are more strongly associated with acute infection response. Therefore, for older patients with less naïve cells available to respond to acute injury, we hypothesize that there is a stronger dependence on preexisting memory T cells, in contrast to the younger patient population. It is also possible that for the younger patients experiencing infection, the presence of an abundance of antigen-specific TEMRA cells is a marker of advanced immunologic age, representing an acceleration from their observed chronologic age.
Analysis on a single cell level demonstrated that these CD8+ TEMRA cells express transcripts associated with cytotoxic T cells including Granzyme B and granulysin (GNLY) and lack CD28 expression, a marker of senescence [24–25]. We additionally noted expression of transcripts associated with T cell dysfunction, namely LAIR2, ZEB2, and HOPX [16,26–27]. Similarly, these terminally differentiated T cells demonstrated increased expression of receptors KLRD1 and NKG7 commonly found on NK cells, a phenotype associated with CD28− senescent T cells [28]. Patients without infection displayed a different phenotype, with increased frequency of CCR7+ naïve and central memory T cells expressing LEF1, which has been also been postulated as a marker of stem cell memory T cells [29]. This analysis demonstrates overlap with transcripts identified in our previous analysis of older compared with younger kidney transplant recipients, where younger patients demonstrated increased frequency of CCR7, LEF1, and CD27 genes [30].
This high-level profiling of the specific T cell dysfunction associated with vulnerability to infection represents one of the first demonstrations of links between T cell attributes associated with infection in patients receiving immunosuppression, and may lead to several potential clinical applications. First, it reveals the mechanism behind vulnerability to infection including CMV in transplant patients despite similar CMV serotype and prophylaxis strategies. It additionally provides a pathway for patient risk stratification through evaluation of T cell phenotype using either a flow cytometry-based or gene expression based approach, suggesting the possibility of altering patient immunosuppression regimens, for example by decreasing target tacrolimus trough levels of dose of mycophenolate mofetil in patients enriched with dysfunctional T cells. Ongoing monitoring of the impact of these changes on frequency of senescent T cells would allow for protection against infection, as well as malignancy, without increasing risk of allograft rejection.
An important aspect of this analysis is the impact of patient age on cytokine expression and protection from infection. For both CMV and SEB antigens, the association between increased frequency of single or double cytokine secreting T cells and protection from infection was more marked in the ≥ 60 year old patient group. This suggests that in the setting of age-associated senescence, ability to respond to antigen stimulation is a more important predictor of protection from infection than in a younger patient population, who possess a higher frequency of naïve T cells. Subsetting these cytokine-secreting T cells by maturation subtype demonstrated that the key subtype of interest that confers this difference is the CD4+ effector memory T cell. This maturation subtype has been shown to be important for infection control after stem cell transplantation, where reconstitution of CD4 effector memory T cells expressing IL-2 has been shown to play a key role in controlling CMV reactivation [31]. It is possible that immunosuppression-driven depletion of peripheral T cells leads to decreases in these protective cell types and impacts infection control. In addition, polyfunctional CMV-specific T cells, expressing two or more cytokines, are known to increase with patient age as well as with repeated antigen exposure [32]. Polyfunctional cells are protective in all patients because these are the most effective CMV-specific cells, which may explain why increased frequency is associated with freedom from infection. This association was more striking for the CMV antigen stimulus as opposed to the more general SEB stimulation. The frequency of monofunctional cells are also decreased in older as compared to younger patients for both CMV and SEB antigen, possibly as a marker of broader immunosenescence as opposed to specifically diminished response to CMV. This may explain why although the mean frequency of T cells displaying a monofunctional antigen response was decreased in the older as compared with the younger patients, the relationship between monofunctional antigen response and infection more similar in both the older and the younger patient cohorts.
Limitations of this analysis include the cohort size, which limits ability to subset patients by age or other clinical characteristics. In addition, T cell analysis is performed only at a single time point, which limits ability to evaluate the impact of immunosuppression on T cell phenotype and how this may impact vulnerability to infection. The flow cytometry panel utilized for detection of secreted cytokines was limited in its ability to fully phenotype T cells responding to antigen stimulation. These limitations will be addressed in future studies that follow a larger cohort of patients before and after transplantation, with several post transplant time points, allowing for a more dynamic assessment of changes in response to antigen stimulation and evolution of the terminally differentiated T cells phenotype.
This multilevel analysis of T cell phenotype and function and association with vulnerability to infection represents an important step forward in our understanding of the mechanism of impaired immune function in transplant recipients. This analysis allows us to develop a biological understanding of immunologic aging in the transplant recipient. Future studies will investigate how these dysfunctional T cell profiles change with the start of immunosuppression, and whether individualized adjustment of immunosuppression can provide biologic and clinical benefit. These questions are especially relevant in the context of an infectious pandemic, where a better understanding of vulnerability to infection and response to vaccination is increasingly important both for the older patient as well as for patients receiving immunosuppression [33].
Supplementary Material
Acknowledgements
The authors wish to thank Victoria Groysberg and Megan Llamas from the UCLA Immunogenetics Center Transplant Biorepository for their laboratory and clinical trial support.
Funding
This study was supported by a National Institutes of Health grant U19 AI128913 (E.F.R.), R03AG050946, R21 AG055879–01A1, and the Mendez National Institute of Transplantation. (J.S.)
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.humimm.2022.01.016.
References
- [1].Meier-Kriesche H-U, Ojo AO, Hanson JA, Kaplan B, Exponentially increased risk of infectious death in older renal transplant recipients, Kidney Internat. 59 (4) (2001) 1539–1543. [DOI] [PubMed] [Google Scholar]
- [2].Dharnidharka VR, Caillard S, Agodoa LY, Abbott KC, Infection frequency and profile in different age groups of kidney transplant recipients, Transplantation 81 (12) (2006) 1662–1667. [DOI] [PubMed] [Google Scholar]
- [3].Linares L, Cofán F, Cervera C, Ricart MJ, Oppenheimer F, Campistol JM, Moreno A, Infection-related mortality in a large cohort of renal transplant recipients, Transplant Proc. 39 (7) (2007) 2225–2227. [DOI] [PubMed] [Google Scholar]
- [4].Cippà PE, Schiesser M, Ekberg H, van Gelder T, Mueller NJ, Cao CA, Fehr T, Bernasconi C, Risk stratification for rejection and infection after kidney transplantation, Clin. J. Am. Soc. Nephrol. 10 (12) (2015) 2213–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Knoll GA, Kidney transplantation in the older adult, YAJKD 61 (5) (2013) 790–797. [DOI] [PubMed] [Google Scholar]
- [6].Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, Gu H, Hirth RA, Hutton D, Jin Y, Kapke A, Kurtz V, Li Y, McCullough K, Modi Z, Morgenstern H, Mukhopadhyay P, Pearson J, Pisoni R, Repeck K, Schaubel DE, Shamraj R, Steffick D, Turf M, Woodside KJ, Xiang J, Yin M, Zhang X, Shahinian V, US renal data system 2019 annual data report: epidemiology of kidney disease in the United States, Am. J. Kidney Dis. 75 (1) (2020) A6–A7. [DOI] [PubMed] [Google Scholar]
- [7].Hart A, Lentine KL, Smith JM, Miller JM, Skeans MA, Prentice M, Robinson A, Foutz J, Booker SE, Israni AK, Hirose R, Snyder JJ, OPTN/SRTR 2019 annual data report: Kidney, Am. J. Transplant. 21 (S2) (2021) 21–137. [DOI] [PubMed] [Google Scholar]
- [8].Poland GA, Ovsyannikova IG, Kennedy RB, Lambert ND, Kirkland JL, A systems biology approach to the effect of aging, immunosenescence and vaccine response, Curr. Opin. Immunol. 29 (2014) 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pera A, Campos C, López N, Hassouneh F, Alonso C, Tarazona R, Solana R, Immunosenescence: Implications for response to infection and vaccination in older people, Maturitas 82 (1) (2015) 50–55. [DOI] [PubMed] [Google Scholar]
- [10].Wong GCL, Strickland MC, Larbi A, Changes in T cell homeostasis and vaccine responses in old age, Interdiscip. Top Gerontol. Geriatr. 43 (2020) 36–55. [DOI] [PubMed] [Google Scholar]
- [11].Zhou D, Borsa M, Simon AK, Hallmarks and detection techniques of cellular senescence and cellular ageing in immune cells, Aging Cell 20 (2) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sanderson SL, Simon AK, In aged primary T cells, mitochondrial stress contributes to telomere attrition measured by a novel imaging flow cytometry assay, Aging Cell 16 (6) (2017) 1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Buchholz VR, Busch DH, Back to the future: effector fate during T cell exhaustion, Immunity 51 (6) (2019) 970–972. [DOI] [PubMed] [Google Scholar]
- [14].Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, Tsao H-W, Godec J, LaFleur MW, Brown FD, Tonnerre P, Chung RT, Tully DC, Allen TM, Frahm N, Lauer GM, Wherry EJ, Yosef N, Haining WN, The epigenetic landscape of T cell exhaustion, Science 354 (6316) (2016) 1165–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tu W, Rao S, Mechanisms underlying T cell immunosenescence: aging and cytomegalovirus infection, Front. Microbiol. 7 (2016) 2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Marchi E, Lee LN, Klenerman P, Inflation vs. exhaustion of antiviral CD8+ T-cell populations in persistent infections: two sides of the same coin?, Front Immunol. 10 (2019) 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schaenman JM, Rossetti M, Sidwell T, Groysberg V, Sunga G, Korin Y, Liang E, Zhou X, Abdalla B, Lum E, Bunnapradist S, Pham T, Danovitch G, Reed EF, Increased T cell immunosenescence and accelerated maturation phenotypes in older kidney transplant recipients, Hum. Immunol. 79 (9) (2018) 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nikolich-Žugich J, van Lier René.A.W., Cytomegalovirus (CMV) research in immune senescence comes of age: overview of the 6th International Workshop on CMV and Immunosenescence, Geroscience. 39 (3) (2017) 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Klenerman P, Oxenius A. T cell responses to cytomegalovirus. 2016:1–11. [DOI] [PubMed] [Google Scholar]
- [20].Liang EC, Rossetti M, Sidwell T, Groysberg V, Sunga G, Korin Y, Vangala S, Abdalla B, Lum E, Bunnapradist S, Pham P-T, Danovitch G, Reed EF, Schaenman J, Differences in proinflammatory cytokines and monocyte subtypes in older as compared with younger kidney transplant recipients, Transplantation Direct. 4 (3) (2018) e348, 10.1097/TXD.0000000000000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ, Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects, J. Exp. Med. 202 (5) (2005) 673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schaenman JM, Korin Y, Sidwell T, Kandarian F, Harre N, Gjertson D, Lum EL, Reddy U, Huang E, Pham PT, Bunnapradist S, Danovitch GM, Veale J, Gritsch HA, Reed EF, Increased frequency of BK virus-specific polyfunctional CD8+ T cells predict successful control of BK viremia after kidney transplantation, Transplantation 101 (6) (2017) 1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schaenman JM, Rossetti M, Liang EC, Lum E, Abdalla B, Bunnapradist S, Pham PT, Danovitch G, Reed EF, Cole SW, Leukocyte transcriptome indicators of development of infection in kidney transplant recipients, Clin. Transplant. 35 (4) (2021), 10.1111/ctr.v35.410.1111/ctr.14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang H, Weyand CM, Goronzy Jörg.J., Hallmarks of the aging T-cell system, FEBS J. 288 (24) (2021) 7123–7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rodriguez IJ, Lalinde Ruiz N, Llano León M, Martínez Enríquez L, Montilla Velásquez María.del.P., Ortiz Aguirre JP, Rodríguez Bohórquez OM, Velandia Vargas EA, Hernández ED, Parra López CA, Immunosenescence study of T cells: A systematic review, Front. Immunol. 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Peng DH, Rodriguez BL, Diao L, Chen L, Wang J, Byers LA, Wei Y, Chapman HA, Yamauchi M, Behrens C, Raso G, Soto LMS, Cuentes ERP, Wistuba II, Kurie JM, Gibbons DL, Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8(+) T cell exhaustion, Nat. Commun. 11 (1) (2020) 4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wirth TC, Xue H-H, Rai D, Sabel JT, Bair T, Harty JT, Badovinac VP, Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8+ T cell differentiation, Immunity 33 (1) (2010) 128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Weng N.-ping., Akbar AN, Goronzy J, CD28– T cells: their role in the age-associated decline of immune function, Trends Immunol. 30 (7) (2009) 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E, The who’s who of T-cell differentiation: Human memory T-cell subsets, Eur. J. Immunol. 43 (11) (2013) 2797–2809. [DOI] [PubMed] [Google Scholar]
- [30].Schaenman JM, Rossetti M, Lum E, Abdalla B, Bunnapradist S, Pham T-P, Danovitch G, Reed EF, Cole S, Differences in gene expression in older compared with younger kidney transplant recipients, Transplant Direct. 5 (4) (2019) e436, 10.1097/TXD.0000000000000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pourgheysari B, Piper KP, McLarnon A, Arrazi J, Bruton R, Clark F, Cook M, Mahendra P, Craddock C, Moss PAH, Early reconstitution of effector memory CD4+ CMV-specific T cells protects against CMV reactivation following allogeneic SCT, Bone Marrow Transplant. 43 (11) (2009) 853–861. [DOI] [PubMed] [Google Scholar]
- [32].Chiu YL, Lin CH, Sung BY, Chuang YF, Schneck JP, Kern F, Pawelec G, Wang GC, Cytotoxic polyfunctionality maturation of cytomegalovirus-pp65-specific CD4 + and CD8 + T-cell responses in older adults positively correlates with response size, Sci. Rep. 6 (2016) 19227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chen Y, Klein SL, Garibaldi BT, Li H, Wu C, Osevala NM, Li T, Margolick JB, Pawelec G, Leng SX, Aging in COVID-19: Vulnerability, immunity and intervention, Ageing Res Rev. 65 (2021) 101205, 10.1016/j.arr.2020.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


