Figure 2. SxtA MT-DC catalysis and structure.
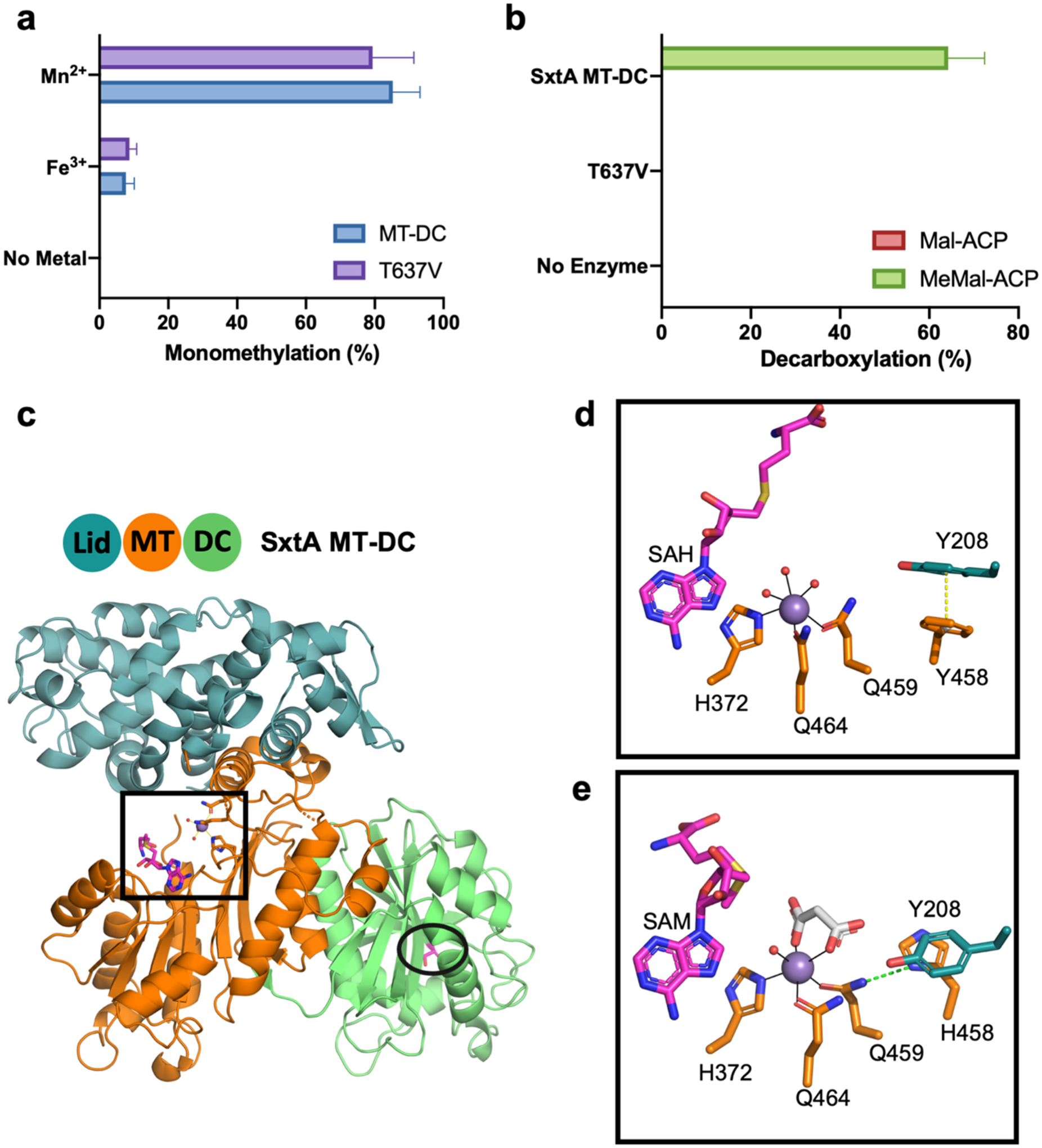
(a) SxtA MT-DC wild type and T637V methylation with added Mn2+, Fe3+ and no-metal control. Monomethylation was quantitated as percent of total Mal-ACP substrate converted to methylated products; no dimethylation products were detected. (b) SxtA MT-DC wild type and T637V decarboxylation with Mal-ACP and MeMal-ACP substrates. Decarboxylation was quantitated as percent of total Mal-ACP or MeMal-ACP substrate converted to acetyl- or propionyl-ACP. Error bars represent the standard deviation of >5 experiments. (c) Didomain ternary complex with Mn2+ (purple), SAH and Thr637 (magenta C) with domain coloring (blue MT lid, orange MT core, green DC). Active sites are boxed (MT) and circled (DC) in stick form (red O, blue N, yellow S). (d) SxtA MT active site with stacked Phe458 and Tyr208. SAH homocysteine is modeled; side chain C colored by domain of origin; waters in red. (e) MT active site of SxtA F458H. Malonate (white C) replaces two water ligands; SAM is modeled. His458 is hydrogen bonded to Gln459 ligand (dashed line).
