Abstract
Endothelial progenitor cells (EPCs) are reduced in number and impaired in function in diabetic patients. Whether and how Nrf2 regulates the function of diabetic EPCs remains unclear. In this study, we found that the expression of Nrf2 and its downstream genes were decreased in EPCs from both diabetic patients and db/db mice. Survival ability and angiogenic function of EPCs from diabetic patients and db/db mice also were impaired. Gain- and loss-of-function studies, respectively, showed that knockdown of Nrf2 increased apoptosis and impaired tube formation in EPCs from healthy donors and wild-type mice, while Nrf2 overexpression decreased apoptosis and rescued tube formation in EPCs from diabetic patients and db/db mice. Additionally, proangiogenic function of Nrf2-manipulated mouse EPCs was validated in db/db mice with hind limb ischemia. Mechanistic studies demonstrated that diabetes induced mitochondrial fragmentation and dysfunction of EPCs by dysregulating the abundance of proteins controlling mitochondrial dynamics; upregulating Nrf2 expression attenuated diabetes-induced mitochondrial fragmentation and dysfunction and rectified the abundance of proteins controlling mitochondrial dynamics. Further RNA-sequencing analysis demonstrated that Nrf2 specifically upregulated the transcription of isocitrate dehydrogenase 2 (IDH2), a key enzyme regulating tricarboxylic acid cycle and mitochondrial function. Overexpression of IDH2 rectified Nrf2 knockdown- or diabetes-induced mitochondrial fragmentation and EPC dysfunction. In a therapeutic approach, supplementation of an Nrf2 activator sulforaphane enhanced angiogenesis and blood perfusion recovery in db/db mice with hind limb ischemia. Collectively, these findings indicate that Nrf2 is a potential therapeutic target for improving diabetic EPC function. Thus, elevating Nrf2 expression enhances EPC resistance to diabetes-induced oxidative damage and improves therapeutic efficacy of EPCs in treating diabetic limb ischemia likely via transcriptional upregulating IDH2 expression and improving mitochondrial function of diabetic EPCs.
Keywords: Endothelial progenitor cell, Nuclear factor erythroid 2-related factor 2, Angiogenesis, Hind limb ischemia, Diabetes
1. Introduction
Microvascular and macrovascular complications associated with diabetes mellitus are leading causes of morbidity and mortality for diabetic patients. These vascular complications are associated with dysregulation of vascular remodeling and vascular growth, decreased responsiveness to ischemic/hypoxic stimuli, impaired or abnormal neovascularization, and a lack of endothelial regeneration under diabetic conditions, indicating that promoting angiogenesis is a promising strategy for the therapy of diabetic vascular complications [1]. Endothelial progenitor cells (EPCs), precursor of endothelial cells, are considered to play critical roles in physiological angiogenesis and endothelium homeostasis [2]. EPCs facilitate angiogenesis by directly incorporating into ischemic sites to form neo-vessels [3], and/or operate in a paracrine fashion by secreting proangiogenic factors [4]. Local or systemic administration of EPCs from bone marrow (BM) [5], cord blood [6] or peripheral blood [7] can enhance ischemic neovascularization and improve function of ischemic tissues in animals with hind limb ischemia (HLI) or myocardial infarction. However, the number of circulating EPCs is decreased and function is impaired in both type 1 [8] and type 2 diabetes [9], contributing to attenuated angiogenesis and delayed repair of the injured endothelium. Several explanations for diabetic EPC dysfunction have been proposed [10], including excessive generation of reactive oxygen species (ROS) [11], over-production of oxidized low density lipoprotein (LDL) [12], defective nitric oxide pathway [13], impaired EPC mobilization [14], and aggravated inflammation [15]. Although several medications have been tested to promote the function of diabetic EPCs, positive clinic outcomes of these treatments are limited [16]. It is unlikely that EPC dysfunction under diabetic conditions is a consequence of a single independent mechanism, and thus, a more complete understanding of the underlying defects is required in order to ameliorate EPC dysfunction and improve therapeutic strategies for diabetic vascular complications.
Our previous study demonstrated that impaired stromal cell-derived factor 1/C-X-C chemokine receptor type 7 (CXCR7) signaling is associated with EPC dysfunction under diabetic conditions. Elevating CXCR7 enhances EPCs resistance to diabetes-induced oxidative injury and improves the therapeutic efficacy of EPCs for diabetic ischemia treatment via activating nuclear factor erythroid 2-related factor 2 (Nrf2) signal pathway [3]. Nrf2 is a basic region-leucine zipper-type transcription factor belonging to the Cap ‘n’ collar (CNC) family. It can translocate and accumulate in the nucleus and bind to the antioxidant responsive element (ARE) [17] where it mediates the expression of more than 200 cytoprotective genes, including antioxidant proteins, phase I and II detoxification enzymes, transport proteins, proteasome subunits, chaperones, growth factors and their receptors, and other transcription factors [18]. As a stress-responsive transcription factor, Nrf2 is the primary master of the inducible cell defense system. Whether and how Nrf2 serves a direct role in regulating the survival and function of EPCs under diabetic conditions remains unclear.
Mitochondria play an essential role in the maintenance of normal cellular functions. Mitochondrial dysfunction is a key mediator of impaired EPC function under hypertensive conditions [19]. Moreover, mitochondrial dysfunction increases electron leak from the mitochondrial respiratory chain and upregulates mitochondrial ROS (mtROS) production [20], which are well-known inducers of impaired reendothelialization and angiogenic capacity of EPCs under diabetic conditions [21]. To maintain mitochondrial quality and homeostasis, mitochondrial morphologies and metabolic status change rapidly in response to external insults through fusion and fission dynamics [22,23]. Mitochondrial fusion creates large mitochondria, while mitochondrial fission produces small mitochondria. The frequency of mitochondrial fusion and fission is dynamically balanced in healthy cells. However, under pathological conditions, significantly smaller or larger mitochondria have been observed. For example, exposure to excess nutritional environment, such as diabetes or obesity, promotes mitochondrial fission and decreases mitochondrial fusion, resulting in mitochondrial fragmentation and uncoupled respiration [24,25]. It remains largely unknown whether an altered mitochondrial size or imbalanced activities of fission or fusion contributes to EPC dysfunction induced by diabetes.
Given that Nrf2 activation is positively associated with mitochondrial biogenesis and mitochondrial quality control [26], we hypothesized that Nrf2 would improve angiogenic function of diabetic EPCs through improvement of mitochondrial dynamics. Thus, the aims of the present study were to investigate whether Nrf2 could rescue EPC dysfunction in diabetes and improve the angiogenic function of EPCs in diabetic limb ischemia and to reveal the possible mechanisms connecting EPC function and mitochondrial dynamics.
2. Materials and methods
2.1. Subject characteristics
The patients suffering from type 2 diabetes (n = 8) were recruited from the First Affiliated Hospital of Chengdu Medical College (Chengdu, China). Peripheral blood (20 ml) was obtained from each participant prior to treatment. Written informed consent was obtained from all the study participants. Healthy individuals (n = 8) admitted to the same hospital only for preventive examinations were enrolled as controls. The baseline characteristics were shown in Table S1. The study protocol was approved by the Research Ethics Committee of the First Affiliated Hospital of Chengdu Medical College.
2.2. Animals
db/db male mice and their sex-matched littermate wild type (WT) control mice at age of 10–12 weeks were used in this study. All mice were housed in a 12:12-h light-dark cycle and specific pathogen-free facility. All animal procedures were approved by the Animal Policy and Welfare Committee of Chengdu Medical College or the Institutional Animal Care and Use Committee of the University of Louisville, which conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (publication No. 85–23, revised 2011).
2.3. Isolation and culture of human EPCs from peripheral blood
EPCs were isolated from peripheral blood of healthy donors (Healthy-EPC) and diabetic patients (DM-EPC) according to protocols described in our previous studies [3,27,28]. Briefly, blood samples (20 ml) were collected with heparin vacuum blood collection tube (BD Biosciences, San Jose, CA), and mononuclear cells (MNCs) were isolated by density gradient centrifugation using histopaque-1077 (Sigma-Aldrich, St. Louis, MO) within 2 h of sampling. MNCs were suspended in endothelial growth factor-supplemented media (EGM-2 bullet kit, Lonza, Basel, Switzerland) with 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA) and seeded into 6-well plates precoated with 50 μg/mL fibronectin (Sigma-Aldrich, St. Louis, MO). Cells were maintained at 37 °C with 5% CO2 in a humidified incubator. After 3 days of incubation, dead cells were washed away with phosphate buffered saline (PBS) and new medium was added. Medium was changed daily for 7 days, and then every 3 days. After 2 weeks of culture, late EPCs were harvested for identification as described in our previous studies [3,27,28] and used for the following experiments.
2.4. Mouse BM-EPC isolation and culture
EPCs were isolated from BM of WT (WT-EPCs) and db/db (db/db-EPCs) mice using density gradient centrifugation according to protocol described in our previous studies [3,29]. Briefly, BM of femurs and tibias were flushed twice using Hank's balanced salt solution (Thermo Fisher Scientific, Waltham, MA). MNCs were collected by density gradient centrifugation using histopaque-1083 (Sigma-Aldrich, St. Louis, MO) and seeded into 6-well plates precoated with 50 μg/mL fibronectin (Sigma-Aldrich, St. Louis, MO). Cells were maintained in EGM-2 bullet kit with 10% FBS at 37 °C with 5% CO2 in a humidified incubator. After 7 days of culture, early EPCs were characterized as described in our previous studies [3,27] and used for the following experiments.
2.5. Lentiviral vector construction, virus production, and transfection
To upregulate Nrf2 expression in EPCs from diabetic patients or db/db mice, the lentivirus vector containing sequences encoding human or mouse Nrf2 gene (Lv-Nrf2) was generated by Genechem (Shanghai, China). In addition, human isocitrate dehydrogenase 2 (IDH2) overexpression lentivirus vector (Lv-IDH2) was constructed to upregulate IDH2 expression in EPCs. The empty lentivirus vector was used as control (Lv-Ctrl). Human or mouse EPCs were transfected with the purified lentivirus carrying recombinant Nrf2, IDH2 or control vector overnight at multiplicity of transfection (MOI) of 50 with 5 μg/mL polybrene (Genechem, shanghai, China), and the medium was replaced with fresh growth medium 24 h after transfection. After transfection for 72 h, the expression of Nrf2 and IDH2 was detected by quantitative real-time PCR (qRT-PCR) and Western blot assay.
To knockdown Nrf2 expression in EPCs from healthy subjects or WT mice, EPCs were transfected with the lentiviruses containing shRNA against Nrf2 (Nrf2-shRNA) constructed by Genechem (Shanghai, China). Meanwhile, lentiviruses containing shRNA against IDH2 (IDH2-shRNA) was used to knockdown IDH2 expression in EPCs. The lentiviruses containing nonsense shRNA (Ctrl-shRNA) was used as control.
The target sequences for Nrf2 or IDH2 and nonsense sequence as follows:
Human Nrf2-shRNA: 5′-GTCCAAAGAGCAGTTCAATGA-3′;
Mice Nrf2-shRNA: 5′-GCCTTACTCTCCCAGTGAATA-3′;
Human IDH2-shRNA: 5′-GCTGTACATGAGCACCAAGAA-3′;
Nonsense shRNA: 5′-TTCTCCGAACGTGTCACGT-3′;
Transfection was performed following the procedure described above. After transduction for 72 h, the expression of Nrf2 and IDH2 was determined by qRT-PCR and Western blot assay.
2.6. Apoptosis assay
EPCs were seeded on 6-well plates (1 × 105 cells/well) and maintained under culture conditions detailed in figure legends. The non-adherent cells were removed by washing with PBS. Subsequently, adherent cells were released with 0.25% trypsin without EDTA. EPCs were collected by centrifugation and stained with APC-conjugated Annexin V Apoptosis Detection Kit with propidium iodide (PI), according to the manufacturer's instructions (KeyGen BioTech, Nanjing, China). The apoptotic EPCs were detected by a flow cytometry (BD Accuri C6, San Jose, CA; or Beckman Coulter Cytoflex S, Krefeld, Germany). Early apoptotic cells were defined as AnnexinV+/PI−.
2.7. Angiogenesis assay in vitro
The in vitro angiogenic capability of EPCs was determined by Matrigel tube formation assay. Briefly, 96-well plates were coated with growth factor-reduced Matrigel (100 μL/per well, BD Biosciences). EPCs were plated (2 × 104 cells/well) in 100 μL under culture conditions detailed in figure legends and incubated at 37 °C with 5% CO2 for 12 h to form tubes. Images of tubes in each well were taken using an inverted microscopy (Nikon Eclipse E600, Nikon, Kanagawa, Japan). The tube lengths were calculated by Image J software (NIH, Bethesda, MD).
2.8. Quantification of mitochondrial morphology
EPCs were seeded and grown in glass-bottom dishes. Mitochondrial morphology was visualized by incubating the cells with 200 nmol/L MitoTracker Red CMXRos probe (Life Technologies, Carlsbad, CA) for 30 min at 37 °C. Images were acquired using a confocal microscopy (LSM880NLO FLIM, Zeiss, German). The degree of mitochondrial interconnectivity (perimeter2/4*π *area) as a measure of both length and degree of branching was calculated using ImageJ software as described in previous reports [30,31]. A degree of interconnectivity of 1 corresponds to a circular and unbranched mitochondrion, whereas a higher degree of interconnectivity indicates a longer and more branched mitochondrion [31].
2.9. Assay of mitochondrial membrane potential (ΔΨm)
The ΔΨm of EPCs was determined by tetramethylrhodamine methyl ester (TMRM, Ex/Em 548/574 nm, Thermo Fisher Scientific, Waltham, MA) according to the instructions. Briefly, EPCs were rinsed twice with PBS and incubated with 100 nmol/L TMRM for 30 min, then cells were washed twice with PBS and detected by BD Accuri C6 flow cytometry.
2.10. mtROS determination
mtROS production of EPCs was measured by staining with MitoSOX™ Red mitochondrial superoxide indicator (Ex/Em 510/580 nm, Thermo Fisher Scientific, Waltham, MA). Briefly, EPCs were washed twice with PBS, and then incubated with 5 μmol/L MitoSox for 30 min, after which the cells were washed twice with PBS and analyzed with flow cytometry.
2.11. Determination of oxidative stress
To detect the ROS level of EPCs, dihydroethidium (DHE; Molecular Probes, Eugene, OR) probe was used to stain EPCs. DHE is cell permeable and able to react with superoxide to form ethidium, which in turn intercalates with DNA and produces nuclear fluorescence. EPCs were seeded on 24-well plates for 24 h, and then incubated with 5 μmol/L DHE in PBS for 30 min at 37 °C. Nuclear DHE positive staining indicates superoxide generation in cells. The fluorescence intensity was detected by a flow cytometry.
2.12. ATP extraction and quantification
ATP content in EPCs was measured by an ATP Measurement Kit (Beyotime Biotechnology, Shanghai, China) according to the manufacture's instruction. In brief, EPCs were plated in a 6-well plate, ATP was extracted with 200 μL ATP lysis buffer and detected by a luminometer. The concentration of ATP was normalized by the protein concentration measured with a BCA protein assay kit (Beyotime Biotechnology).
2.13. qRT-PCR
Total RNA was extracted using Trizol reagent (RNA STAT 60, Tel-Test Inc., Austin, TX) and reverse transcribed using a GoScript™ Reverse Transcription System (Promega, Madison, WI) following the manufacturer's protocol. The expression of mRNAs was measured by quantitative real-time polymerase chain reaction (qRT-PCR) using the SYBR-Green method (Go Taq® qPCR, Promega Corporation, USA). All the primers used for qRT-PCR were designed and synthesized by Tsingke Biotechnology Co., Ltd. (Shanghai, China). qRT-PCR was performed in duplicate with a 10 μL reaction system, which contained 5 μL SYBR Green PCR Master Mix, 4 μL cDNA, and 1 μL primer, using the ABI 7500 RT-PCR system (Applied Biosystems, Foster City, CA). The comparative cycle time (Ct) method was used to determine fold differences between samples, and the amount of target genes was normalized to β-actin as an endogenous reference (2−△△Ct).
2.14. RNA-sequencing (RNA-Seq) assay
Total RNA was isolated from EPCs transduced with Lv-Ctrl (n = 3), Lv-Nrf2 (n = 3), Ctrl-shRNA (n = 3), or Nrf2-shRNA (n = 3), respectively, using total RNA isolation kit (Solarbio, Beijing, China). The purity and concentration of isolated RNA were determined by a NanoPhotometer spectrophotometer (IMPLEN, CA), the RNA integrity was assessed by the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies, CA). RNA sequencing libraries were constructed using NEBNext Ultra RNA Library Prep Kit for Illumina (New England BioLabs, Ipswich) and index codes were added to attribute sequences to each sample. Clusters were generated using TruSeq PE Cluster Kit v3-cBit-HS (Illumina, San Diego, CA), and RNA-seq libraries were sequenced on an Illumina platform at the Novegene Company (Beijing, China). Raw data (raw reads) of fastq format were initially processed through in-house perl scripts. Gene expression was quantified using HTSeq v0.9.1. Differential expression analysis was performed using the DESeqR package (1.18.0). DESeq provided statistical routines for determining differential expression in digital gene expression data using a model based on the negative binomial distribution. Genes with a P < 0.05 found by DESeq were assigned as differentially expressed.
2.15. Chromatin immunoprecipitation (ChIP) assay
ChIP was performed using a Simple ChIP Plus Enzymatic Chromatin IP Kit (Cell Signaling Technology, Danvers, MA) following the protocol provided by the manufacturer. In brief, EPCs were fixed with formaldehyde, and the chromatin was sheared. Then, the fragmented chromatin was incubated with Nrf2 antibody (Abcam, Cambridge, MA) and protein G magnetic beads. DNA released from the precipitates was analyzed by PCR. IgG was employed as the negative control. The primer sequences specific to the Nrf2 binding region within the IDH2 promoter region were as follows: IDH2 promoter forward: 5′-GACTTGGTGAGGGGAGCTTTGAA-3′ and reverse: 5′-CTGAGGGCTGGGTTTTATTTGGA-3′. As a positive control, the primer sequences specific to the region of NQO1 gene promoter that contains an ARE binding site were as follow: forward primer: 5′-TGCACCCAGGGAAGTGTGTTGTAT-3′ and reverse primer: 5′-CCCTTTTAGCCTTGGCACGAAA-3′. As a negative control, the primer sequences to the region of NQO1 that does not contain an ARE binding site as follow: forward primer: 5′-TCTCAGTTTTTGCCCTTATTTAATC-3′ and reverse primer: 5′-TAAAAAGTAGAGTGGTTGGAGTGATGAC-3′.
2.16. Dual luciferase reporter assay
For reporter assay, the human IDH2 promoter sequences -2000 bp ∼ 0 bp and -1000 bp ∼ 0 bp were cloned into the pGL3.0-Basic plasmid (provided by Tsingke Biotechnology) and designated as IDH2-Full-Luc and IDH2-Short-Luc, respectively. Then the putative binding sites (GTGAATTAGCG) of Nrf2 in IDH2-Full-Luc plasmid was mutated to a non-specific sequence (TATCATTAAAG) by using KOD-Plus-Mutagenesis kit (SMK-101, Toyobo, Osaka, Japan) and designated as IDH2-Mutant-Luc.
Luciferase reporter assays were performed as described previously [32]. To detect whether knockdown of Nrf2 expression inhibiting IDH2 promoter activity, IDH2-Full-Luc, IDH2-Short-Luc, or IDH2-mutant-Luc reporters were co-transfected with pLKO.1-Ctrl-shRNA or pLKO.1-Nrf2-shRNA vector into HEK-293T cells, respectively. Renilla luciferase reporter plasmid (pRL-TK) was used as an internal control of transfection. In addition, to investigate whether activation of Nrf2 promoting IDH2 promoter activity, we used Nrf2 activator sulforaphane (SFN) to treat HEK-293T cells transfected with IDH2-Full-Luc, IDH2-Short-Luc, or IDH2-mutant-Luc reporters, respectively. Cell lysates were subjected to luciferase assay by using a GloMax 96 microplate luminometer (Promega, Madison, WI) at 48 h post-transfection. For each sample, the pGL3.0-firefly luciferase activity was normalized to Renilla luciferase activity of the pRL-TK control.
2.17. Western blot assay
Western blot was performed as described in previous studies [3,27,29]. For cellular protein extraction, EPCs were rinsed twice with PBS and then suspended in ice-cold RIPA lysis buffer (Solarbio Life Sciences, Beijing, China) and incubated for 30 min on ice. Proteins were collected by centrifugation, 12,000 g for 15 min at 4 °C. The protein concentration was determined using a BCA protein assay kit. The proteins were separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). The membranes were blocked in tris-buffered saline with 5% non-fat milk and 0.5% BSA for 1 h, and then incubated with primary antibody overnight at 4 °C, followed by incubation with the secondary antibody for 1 h at room temperature after standard washing procedures. The primary antibodies against Nrf2 (1:1000), mitofusin 1 (Mfn1, 1:1000), NAD(P)H dehydrogenase quinone 1 (NQO-1, 1:1000), optic atrophy1 (Opa1, 1:1000) were purchased from Abcam (Cambridge, MA); heme oxygenase-1 (HO-1, 1:1000), mitofusin 2 (Mfn2, 1:1000) and IDH2 (1:1000) were purchased from Cell Signaling Technology; β-actin (1:2000) was purchased from Bioss Biotechnology; dynamic related protein 1 (Drp1, 1:1000) was purchased from Novus Biologicals (Littleton, CO); mitochondrial fission 1 protein (Fis1, 1:1000) was purchased from GeneTex (San Antonio, TX). All horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Bioss Biotechnology (Beijing, China). Blots were visualized with Chemiluminescent HRP Substrate (Millipore, Billerica, MA) and quantified with Quantity 5.2 software System (Bio-Rad).
2.18. HLI model and cell therapy
db/db type 2 diabetic mice at the age of 10–12 weeks were used to develop HLI model as reported in our previous studies [3]. Briefly, under sufficient anesthesia with isoflurane (1–3% isoflurane in 100% oxygen at a flowrate of 1 L/min), the right hind limb was shaved and the entire right superficial femoral artery and vein (from just below of deep femoral arteries to popliteal artery and vein) were ligated with 6–0 silk sutures, cut, and excised with an electric coagulator (Fine Science Tools Inc., Foster City, CA), and the skin was closed with 4–0 silk sutures. Real time microcirculation imaging analysis was performed using a Pericam Perfusion Speckle Imager (PSI, Perimed Inc., Kings Park, NY) to evaluate the foot pad blood perfusion ratio [ischemic limb (right)/normal limb (left)] at days 0, 3, 7, 14, 21, and 28 post-ischemia [3]. Then mice were euthanized and gastrocnemius muscle and soleus muscle of the ischemia limb were collected for analysis of capillary density.
For cell therapy in db/db mice, 2.5 × 106 WT-EPCs or db/db-EPCs were infused into db/db male mice via tail vein within 1 h after HLI surgery. For SFN treatment, db/db mice were pretreated with SFN (1.0 mg/kg body weight, every other day by intraperitoneal injection) [33,34] for 1 week, followed by HLI surgery and continued SFN treatment for an additional 4 weeks until the end of experiment. SFN was dissolved in 1% dimethyl sulfoxide (DMSO) and diluted in PBS; an equivalent concentration of DMSO in PBS was used as vehicle control. To evaluate whether overexpression IDH2 can rescue the angiogenic dysfunction of mouse EPC induced by Nrf2-knockdown, 2.5 × 106 WT-EPCs co-transfected with lentivirus carrying Ctrl-shRNA and control vector (Ctrl-shRNA/Lv-Ctrl-EPC), Nrf2-shRNA and control vector (Nrf2-shRNA/Lv-Ctrl-EPC), or Nrf2-shRNA and IDH2 over-expression vector (Nrf2-shRNA/Lv-IDH2-EPC) were infused into db/db male mice via tail vein within 1 h after HLI surgery. Foot pad blood perfusion was evaluated by PSI at days 0, 3, 7, 14, 21, and 28 after surgery.
2.19. Histological assessment
Ischemic gastrocnemius muscle and soleus muscle were fixed with 4% paraformaldehyde and embedded with paraffin. Paraffin sections were cut at 5 μm and stained with Alexa Fluor® 594 conjugated isolectin GS-IB4 (Thermo Scientific, Waltham, MA) to evaluate the capillary density. The number of capillaries were calculated in randomly selected fields for a total of 20 different fields ( × 40 magnification) per section and 3 sections per animal. The capillary density was expressed as capillary number per muscle fiber.
2.20. Statistical analysis
All data are presented as mean ± SD. Sample size for each study was detailed in figure legends. Statistical analysis was performed using GraphPad Prism 8.0 software with one-way or two-way ANOVA, followed by post-hoc multiple comparisons with the Scheffe’ test. Statistical significance was considered as p < 0.05.
3. Results
3.1. Diabetes attenuates Nrf2 and impairs the survival and angiogenic function of EPCs
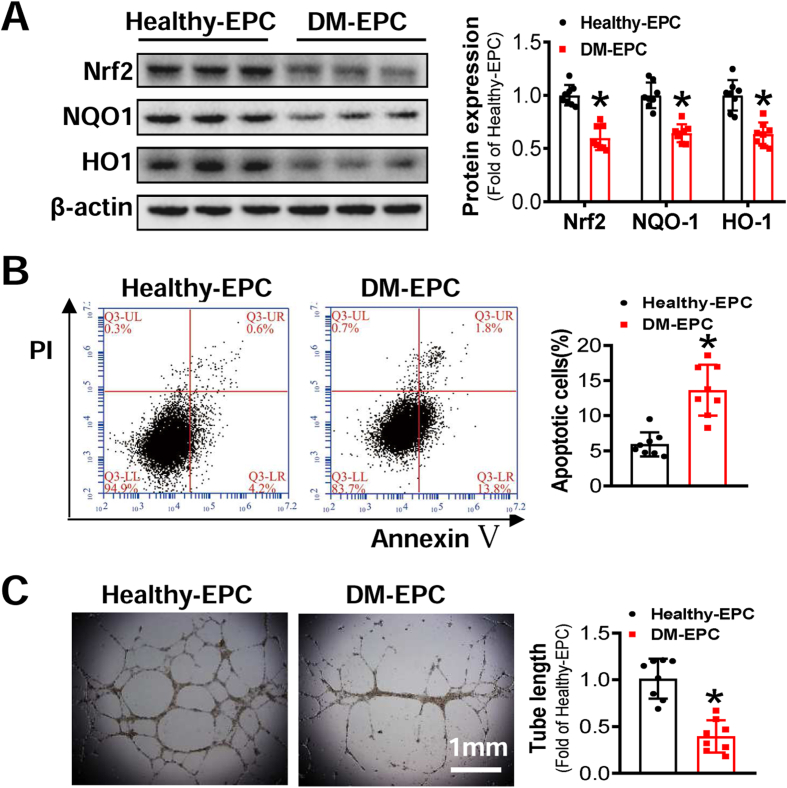
To investigate the role of Nrf2 in EPCs, the expression of Nrf2 and its downstream genes were detected. The results showed that the expression of Nrf2 and its downstream genes was significantly decreased in DM-EPCs, compared with Healthy-EPCs (Fig. 1A). We further investigated the survival and angiogenic function of Healthy-EPCs and DM-EPCs. Annexin V/PI staining showed significantly higher apoptotic percentage of DM-EPCs than that of Healthy-EPCs (Fig. 1B). In addition, tube formation ability of DM-EPCs was also impaired as indicated by the significantly shorter length of tubes produced by DM-EPCs compared with Healthy-EPCs (Fig. 1C). Similarly in mice, the expression of Nrf2 and its downstream genes in db/db-EPCs were lower than that of WT-EPCs (Fig. S1A), and the survival and angiogenic function of db/db-EPCs were also impaired (Figs. S1B and C).
Fig. 1.
Diabetes attenuates Nrf2 and impairs the survival and angiogenic function of EPCs. EPCs were isolated from healthy donors (Healthy-EPC) or diabetic patients (DM-EPC). (A) The Expression of Nrf2 and its downstream genes were detected by Western blot, and the quantitative data was normalized by the average of Healthy-EPCs for each protein; β-actin was used as a loading control. (B) The survival abilities of EPCs were determined by Annexin V/PI staining, and the quantitative data was expressed as the percentage of AnnexinV+/PI– cells. (C) The angiogenic function of EPCs was evaluated by a Matrigel tube formation assay, and the quantitative data was normalized by the average tube length of Healthy-EPCs. n = 8 per group. Data shown in graphs represents the means ± SD. *p < 0.05, vs Healthy-EPC.
3.2. Nrf2 regulates the survival and angiogenic function of EPCs
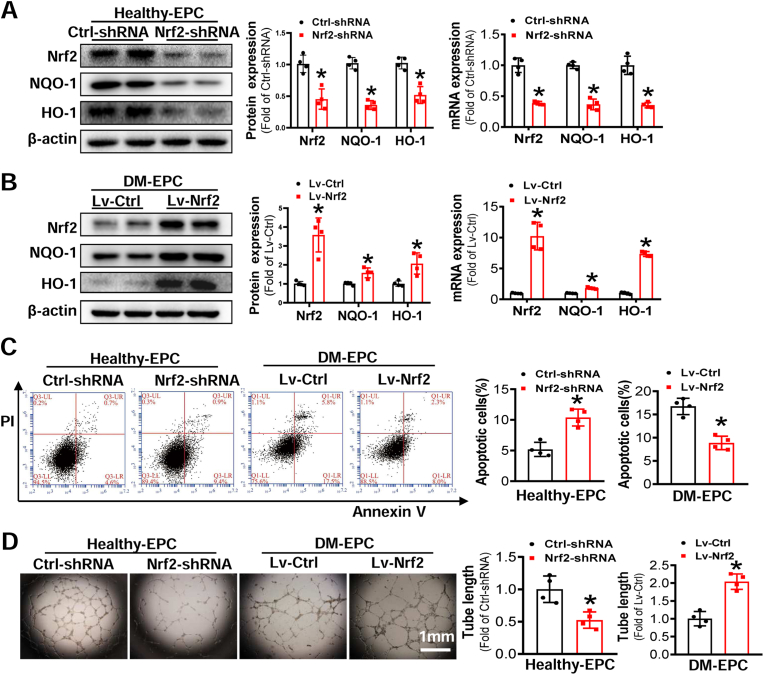
To determine if reduced Nrf2 expression in diabetic EPCs was a cause of angiogenic dysfunction, gain- and loss-of-function studies were performed by Nrf2-shRNA lentivirus to knockdown Nrf2 expression in Healthy-EPCs and WT-EPCs, and Nrf2 overexpression lentivirus to upregulate Nrf2 expression of DM-EPCs and db/db-EPCs, respectively. The expression of Nrf2 and its downstream genes was significantly reduced by the Nrf2-shRNA at mRNA and/or protein levels in Healthy-EPCs (Fig. 2A) and WT-EPCs (Fig. S2A), and this was accompanied by increased apoptosis (Fig. 2C, Fig. S2C) and impaired tube formation (Fig. 2D, Fig. S2D). Conversely, transduction of Lv-Nrf2 into DM-EPCs and db/db-EPCs elevated the expression of Nrf2 and its downstream genes (Fig. 2B, Fig. S2B), diminished cell apoptosis (Fig. 2C, Fig. S2C) and improved tube formation in DM-EPCs and db/db-EPCs (Fig. 2D, Fig. S2D).
Fig. 2.
Downregulation of Nrf2 impairs the survival and anigogenic function of Healthy-EPCs, while upregulation of Nrf2 rescues the survival and angiogenic function of DM-EPCs. Healthy-EPCs were transfected with lentivirus carrying nonsense shRNA (Ctrl-shRNA) or Nrf2 shRNA (Nrf2-shRNA) and DM-EPCs were transfected with lentivirus carrying Nrf2 (Lv-Nrf2) or control vector (Lv-Ctrl). (A-B) The efficiency of Nrf2 knockdown or upregulation was determined by Western blot and qRT-PCR, and the quantitative data was normalized by the average of the respective control group for each protein; β-actin was used as a loading control. (C) The survival abilities were determined by Annexin V/PI staining, and the quantitative data was expressed as the percentage of AnnexinV+/PI– cells. (D) Angiogenic function of EPCs was evaluated by a Matrigel tube formation assay, and the quantitative data was normalized by the average tube length of the respective control group. n = 4 per group. Data shown in graphs represents the means ± SD. *p < 0.05, vs Ctrl-shRNA or Lv-Ctrl.
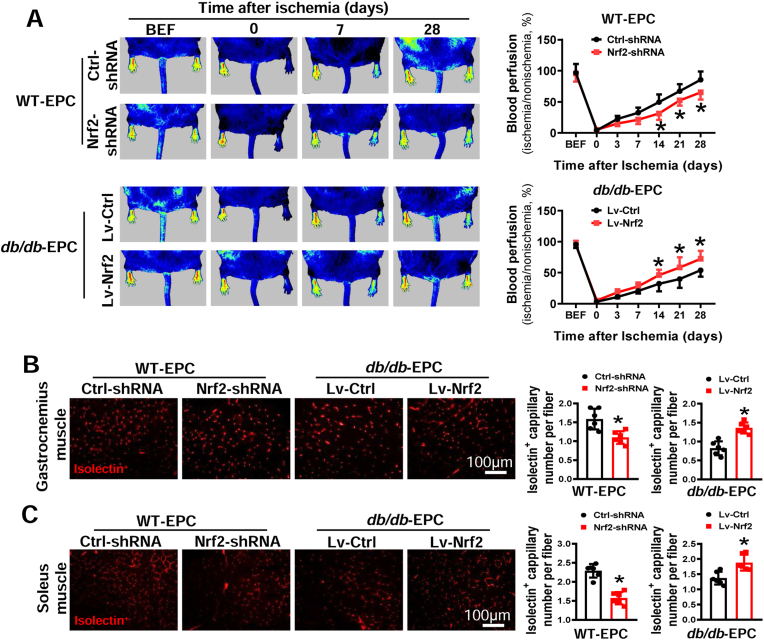
To evaluate the role of Nrf2 in EPC-mediated angiogenesis in vivo, we infused mouse EPCs with manipulated Nrf2 expression into db/db mice with HLI that was induced by femoral artery ligation. As shown in Fig. 3A, infusion of WT-EPCs transfected with Nrf2-shRNA lentivirus showed significantly slower blood flow recovery in db/db mice at days 14, 21 and 28 after HLI surgery compared with infusion of WT-EPCs transfected with Ctrl-shRNA lentivirus. However, elevating Nrf2 expression in db/db-EPCs with Nrf2 overexpression lentivirus remarkably promoted the recovery of blood perfusion in db/db mice compared with infusion of db/db-EPCs transfected with control lentivirus. Furthermore, the capillary density in ischemic muscle was measured at 28 days after HLI. The results demonstrated that knockdown of Nrf2 expression in WT-EPCs reduced the capillary density in both gastrocnemius and soleus muscles (Fig. 3B and C). While upregulating Nrf2 expression in db/db-EPCs significantly increased the capillary density in both gastrocnemius muscle and soleus muscle of db/db mice (Fig. 3B and C). These results suggest that diabetic downregulation of Nrf2 plays a causative role in diabetes-induced EPC angiogenic dysfunction, while elevating Nrf2 expression rescued EPC dysfunction induced by diabetes.
Fig. 3.
Nrf2 regulates the angiogenic function of mouse EPCs. WT-EPCs were transfected with lentivirus carrying Ctrl-shRNA or Nrf2-shRNA and db/db-EPCs were transfected with lentivirus carrying Lv-Ctrl or Lv-Nrf2. Within 1 h after hind limb ischemia (HLI) surgery, db/db mice (FVB background) were transplanted with different types of EPCs that have a same genetic background. (A) The time-course of blood perfusion before (BEF) and after HLI surgery was monitored by a Pericam Perfusion Speckle Imager, and the blood perfusion was quantified by Image J and expressed as the percentage of the perfusion relative to the collateral nonischemic limb. Transverse sections of ischemic gastrocnemius (B) and soleus muscle (C) were stained with Alexa Fluor® 594 conjugated isolectin to enumerate isolectin-stained cell number as a proxy of capillary density. Capillary density was expressed as isolectin+ capillaries per muscle fiber. n = 6 mice per group. Data shown in graphs represents the means ± SD. *p < 0.05, vs Ctrl-shRNA or Lv-Ctrl.
3.3. Diabetes induces mitochondrial dysfunction and ROS overproduction in EPCs
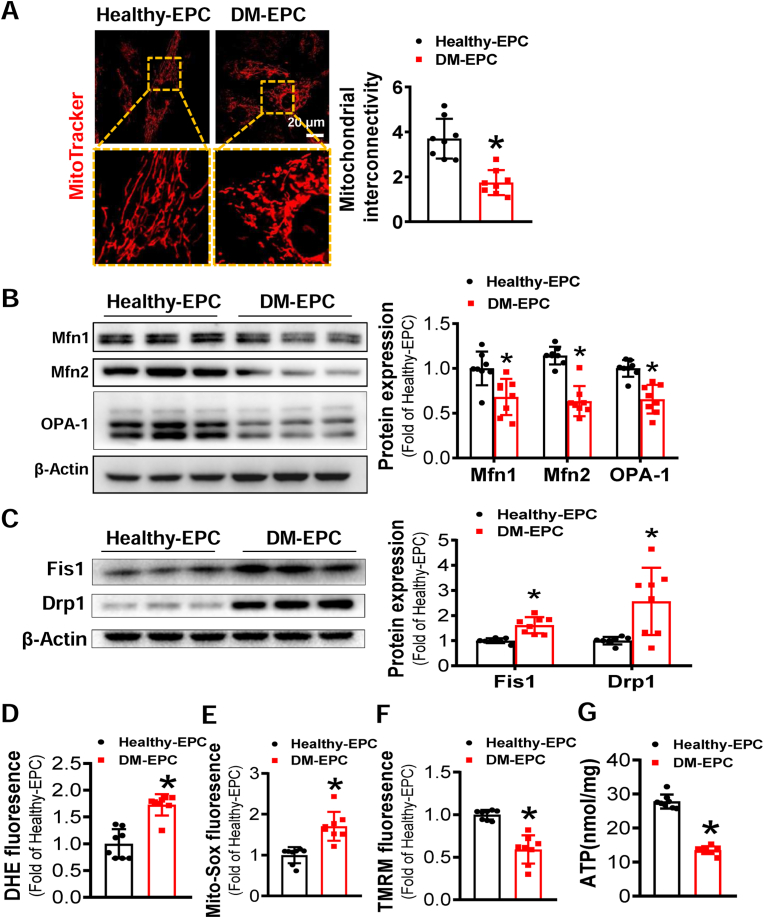
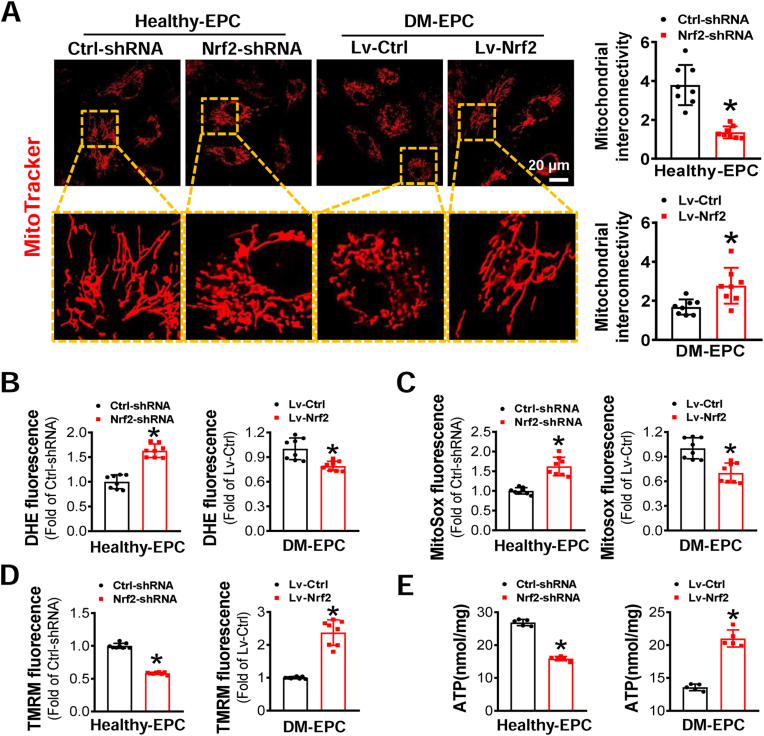
Our previous study showed that oxidative stress is the main cause of diabetes-induced EPC dysfunction [3]. Mitochondria are the major source of ROS generation [35]. Mitochondrial fragmentation is an important contributor to ROS overproduction under diabetic conditions, in which it causes deleterious vascular cell signaling and subsequent endothelial dysfunction [36]. Thus, to uncover whether mitochondrial fragmentation is responsible for the dysfunction of diabetic EPCs, the mitochondrial morphology was visualized by mitochondrial fluorescent probe. As shown in Fig. 4A, most mitochondria in Healthy-EPCs exhibited rod and network organization; however, most mitochondria in DM-EPCs were circular and punctate, indicating mitochondrial fragmentation.
Fig. 4.
Diabetes induces mitochondrial fragmentation of EPCs and impairs mitochondrial function. (A) Micrographs of mitochondrial morphology of EPCs were visualized by MitoTracker Red CMXRos probe staining, and the morphological alterations were quantified by Image J and expressed as mitochondrial interconnectivity. (B–C) The expression of mitochondrial fusion and fission related proteins was determined by Western blot, the quantitative data was normalized by the average of Healthy-EPC for each protein, and β-actin was used as a loading control. The levels of (D) intracellular ROS, (E) mitochondrial ROS, and (F) mitochondrial membrane potential were detected by DHE, MitoSOX™, and TMRM staining, respectively, and quantified by a flow cytometry and normalized by the average fluorescence density of Healthy-EPCs. (G) ATP concentration in EPCs was measured by an ATP assay Kit. n = 8 per group. Data shown in graphs represents the means ± SD. *p < 0.05, vs Healthy-EPC. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The mitochondrial morphological changes reflect dynamics of the mitochondrial network. Several GTPases have been shown to be involved in the regulation of mitochondrial fusion and fission process [24,37]. Specifically, mitochondrial fusion is mainly regulated by Opa1, Mfn1 and Mfn2, and fission is mainly regulated by Drp1 and Fis1 [24,37]. Thus, we detected the abundance of these mitochondrial fusion and fission regulating proteins, respectively. As shown in Fig. 4B, the expression of mitochondrial fusion proteins was lower in DM-EPCs than that in Healthy-EPCs. In contrast, the abundance of mitochondrial fission proteins was higher in DM-EPCs than that in Healthy-EPCs (Fig. 4C). These findings indicate that diabetes impairs mitochondrial remodeling by downregulating the expression of mitochondrial fusion proteins and inducing the expression of mitochondrial fission proteins.
In addition, we also determined the cellular ROS and mtROS production in EPCs by DHE and MitoSOX™ staining, respectively. Both cellular ROS and mtROS were elevated in DM-EPCs relative to Healthy-EPCs (Fig. 4D and E). The ΔΨm was simultaneously monitored with TMRM. As depicted in Fig. 4F, ΔΨm was lower in DM-EPCs than in Healthy-EPCs. The key function of mitochondria is energy production through oxidative phosphorylation; thus, we measured ATP content in both Healthy-EPCs and DM-EPCs. The ATP content in DM-EPCs was lower than that in Healthy-EPCs (Fig. 4G). These findings indicate that diabetes-induced EPC dysfunction is associated with mitochondrial fragmentation and dysfunction.
3.4. Nrf2 attenuates diabetes-induced mitochondrial fragmentation and rectifies the balance of proteins regulating mitochondrial fission and fusion
Based on the above findings, the mitochondrial fragmentation and dysfunction were associated with remarkable mtROS overproduction. Because Nrf2 is a key transcriptional factor regulating cellular redox homeostasis [17], we examined the impact of Nrf2 expression on EPC mitochondrial dynamics and function. As shown in Fig. 5A, following knockdown of Nrf2 expression in Healthy-EPCs, the mitochondria became smaller in size and shorter in length compared with Healthy-EPCs transduced with Ctrl-shRNA. However, the majority of mitochondria in DM-EPCs transduced with Lv-Nrf2 exhibited a longer filamentous and network morphology (Fig. 5A). These results indicate that Nrf2 plays a critical role in regulating mitochondrial dynamics.
Fig. 5.
The effects of Nrf2 expression on mitochondrial dynamics and function of EPCs. Healthy-EPCs were transfected with lentivirus carrying Ctrl-shRNA or Nrf2-shRNA and DM-EPCs were transfected with lentivirus carrying Lv-Ctrl or Lv-Nrf2. (A) Micrographs of mitochondrial morphology were visualized by MitoTracker Red CMXRos probe staining, and the morphological alterations were quantified by Image J and expressed as mitochondrial interconnectivity. The levels of (B) intracellular ROS, (C) mitochondrial ROS, and (D) mitochondrial membrane potential of EPCs were detected by DHE, MitoSOX™, and TMRM staining, respectively, and quantified by a flow cytometry and normalized by the average fluorescence density of the respective control group. (E) ATP concentration in EPCs was measured by an ATP assay Kit. n = 5–8 per group. Data shown in graphs represents the means ± SD. *p < 0.05, vs Ctrl-shRNA or Lv-Ctrl. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
We then assayed intracellular ROS and mtROS in Nrf2-manipunated EPCs as a measurement of mitochondrial efficiency. As expected, knockdown of Nrf2 expression increased ROS and mtROS levels in Healthy-EPCs, while upregulation of Nrf2 expression decreased intracellular ROS and mtROS levels in DM-EPCs (Fig. 5B and C). In addition, knockdown of Nrf2 expression in Healthy-EPCs resulted in decreased ΔΨm; while upregulation of Nrf2 expression in DM-EPCs increased the ΔΨm (Fig. 5D). Similarly, intracellular ATP level was impaired by knockdown of Nrf2 expression in Healthy-EPCs, while upregulation of Nrf2 expression in DM-EPCs increased ATP content (Fig. 5E). These findings support that Nrf2 also plays an essential role in mitochondrial function in EPCs.
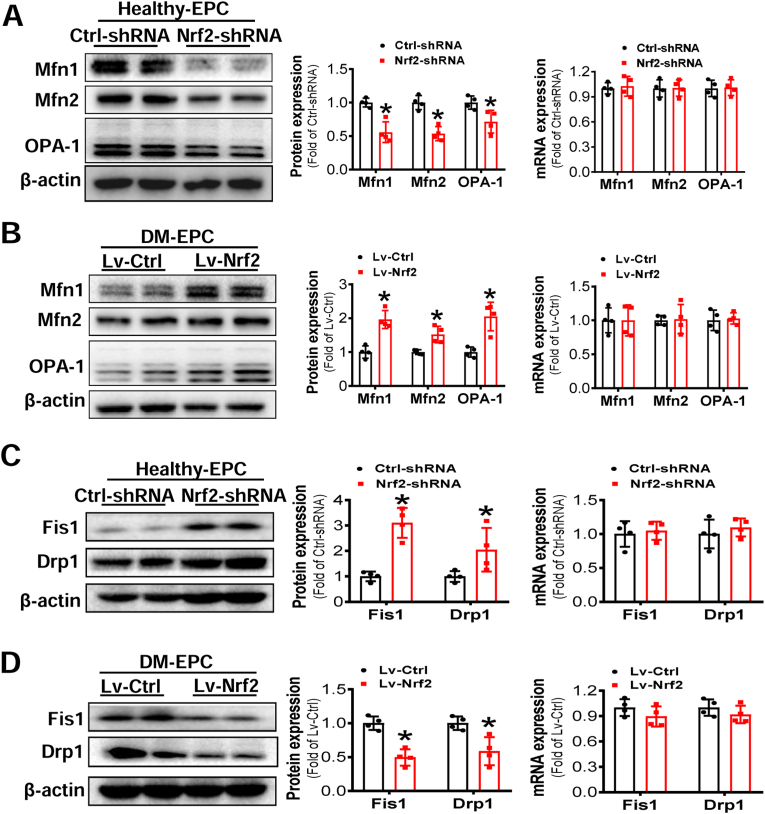
We also examined whether the mitochondrial fusion and fission proteins were affected by Nrf2 expression. Knockdown of Nrf2 expression in Healthy-EPCs significantly decreased the abundance of mitochondrial fusion-related proteins (Mfn1, Mfn2 and Opa1) (Fig. 6A) yet increased the abundance of mitochondrial fission-related proteins (Fis1 and Drp1) (Fig. 6C). Furthermore, upregulation of Nrf2 expression in DM-EPCs significantly increased the abundance of mitochondrial fusion-related proteins (Fig. 6B) and decreased the abundance of mitochondrial fission-related proteins (Fig. 6D). However, neither knockdown of Nrf2 expression in Healthy-EPCs nor upregulation of Nrf2 expression in DM-EPCs had any effects on the mRNA expression of molecular markers related to both mitochondrial fusion and fission (Fig. 6A–D). These findings suggest that Nrf2 may regulate mitochondrial homeostasis indirectly by affecting protein abundance relevant to mitochondrial fission and fusion.
Fig. 6.
Nrf2 expression affected the protein expression of mitochondrial fusion and fission related proteins but not mRNA expression. Healthy-EPCs were transfected with lentivirus carrying Ctrl-shRNA or Nrf2-shRNA and DM-EPCs were transfected with lentivirus carrying Lv-Ctrl or Lv-Nrf2. The expression of mitochondrial fusion (A-B) and fission (C-D) related molecules at protein and mRNA levels were determined by Western blot and q-RT-PCR, respectively; the quantitative data was normalized by the average of the respective control for each molecule, and β-actin was used as a loading control. n = 4 per group. Data shown in graphs represents the means ± SD. *p < 0.05, vs Ctrl-shRNA or Lv-Ctrl.
3.5. Nrf2 suppression of mitochondrial fission improves EPC survival and angiogenic function
To confirm whether suppressing mitochondrial fission plays a pivotal role in Nrf2 protecting diabetic EPC functions, mitochondrial division inhibitor (Mdivi-1) and mitochondrial fission inducer (FCCP) were used to treat Healthy-EPCs and DM-EPCs, respectively. The results showed that Midivi-1 treatment prevented apoptosis (Fig. S3A) and improved the tube formation ability of WT-EPCs transduced with Nrf2-shRNA lentivirus (Fig. S3C). However, in DM-EPCs, FCCP treatment nearly abolished the beneficial actions of Nrf2 upregulation on EPC survival and angiogenic function (Figs. S3B and D). Together, Nrf2 protects survival and angiogenic function of EPCs against diabetes via suppression of mitochondrial fission.
3.6. Nrf2 inhibits mitochondrial fission and improves mitochondrial function via transcriptional upregulation of IDH2
To address how Nrf2 regulates mitochondrial fission and fusion, we performed an RNA-seq assay to screen for genes involved in mitochondrial homeostasis and function in Nrf2-manipunated Healthy-EPCs. We used Healthy-EPCs rather than DM-EPCs to exclude the potential confounding impacts derived from diabetic status in vivo on EPC transcriptome beyond Nrf2 manipulation. Venn diagram showed that there were 234 commonly altered genes between Nrf2 upregulated and downregulated EPCs (Fig. S4A). KEGG (Kyoto Encyclopedia of Genes and Genomes) Pathway Enrichment analysis of Top-10 enriched pathways among these 234 altered genes showed that glutathione metabolism and pentose phosphate pathway were most significantly and positively regulated by Nrf2 (Fig. S4B). Significantly changed representative genes within these metabolic pathways were plotted in a heat-map (Fig. S4C). Among these genes only IDH2 was associated directly with mitochondrial tricarboxylic acid (TCA) cycle and oxidative respiration (Fig. S4C).
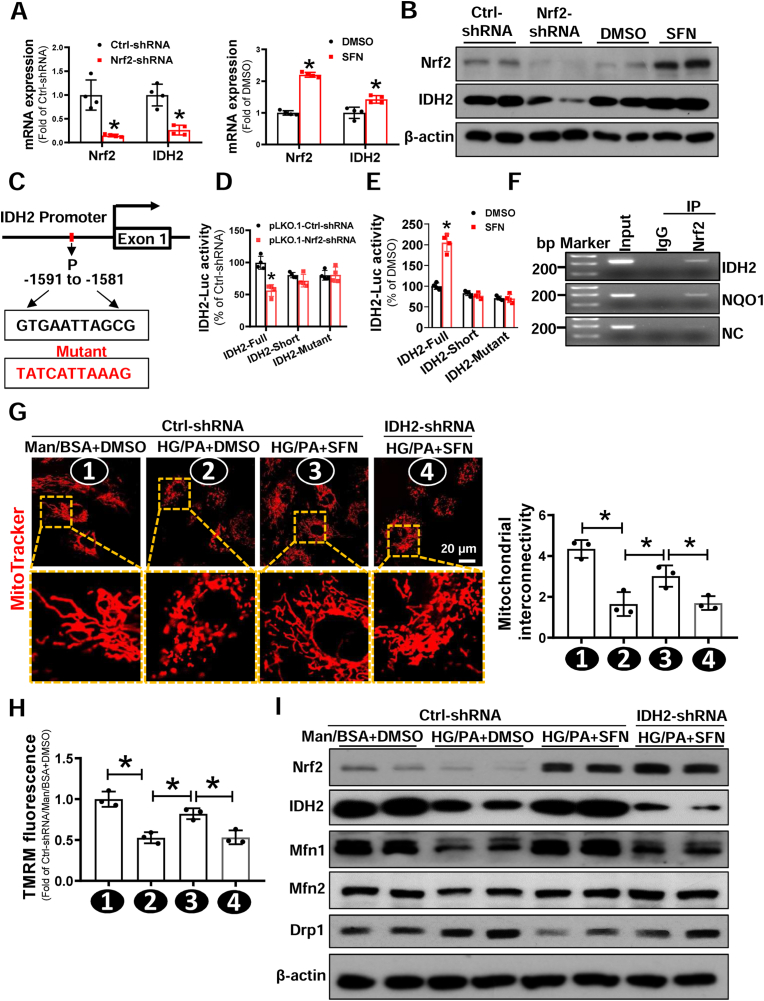
To confirm RNA-seq data, we performed RT-qPCR and immunoblotting assays. knockdown of Nrf2 expression significantly reduced the expression of IDH2 at both mRNA and protein levels in Healthy-EPCs (Fig. 7A and B). Conversely, treatment of Healthy-EPCs with a potent Nrf2 activator, SFN, markedly increased the expression of both Nrf2 and IDH2 (Fig. 7A and B). Furthermore, the protein expression of IDH2 was significantly decreased in both human DM-EPCs and db/db-EPCs (Fig. S5), which was consistent with the expression pattern of Nrf2 in these cells (Fig. 1A and Fig. S1A). These findings imply that Nrf2 inhibits diabetes-induced mitochondrial alterations possibly via transcriptional upregulation of IDH2 expression.
Fig. 7.
Nrf2 suppression of diabetes-induced mitochondrial fission of EPCs is dependent on transcriptional upregulation of IDH2. (A-B) Healthy-EPCs were transfected with lentivirus carrying Ctrl-shRNA or Nrf2-shRNA, or treated with DMSO or Nrf2 activator SFN. The (A) mRNA and (B) protein expression of Nrf2 and IDH2 were determined by Western blot and qRT-PCR, respectively; the quantitative data was normalized by the average of the respective control for each group, and β-actin was used as a loading control. (C) The putative binding sites of Nrf2 on IDH2 promoter was predicted by JASPAR. (D) HEK-293T cells were co-transfected with PLG3.0-IDH2-Luc reporter (Full, Short or mutant) and Nrf2-shRNA or Ctrl-shRNA plasmid. Renilla luciferase reporter plasmid (pRL-TK) was used as an internal control of transfection. Firefly luciferase activity and Renilla luciferase activity in media were measured at 48 h post-transfection. (E) HEK-293T cells were transfected PLG3.0-IDH2-Luc reporter (Full, Short or mutant), then treated with or without SFN, a Nrf2 activator. pRL-TK was used as an internal control of transfection. Firefly luciferase activity and Renilla luciferase activity in media were measured at 48h post-transfection. (F) Chromatin immunoprecipitation (ChIP) analyses using an anti-Nrf2 or a normal rabbit IgG were performed in EPC cells. Primers specific for IDH2, NQO1, or NC (Negative control) were used. (G–I) Healthy-EPCs were transfected with or without lentivirus carrying Ctrl-shRNA or IDH2-shRNA, respectively, followed by exposure to high glucose (HG, 30 mmol/L) plus palmitate (PA, 100 μmol/L) or Ctrl (mannitol plus bovine serum albumin, Man/BSA) in the presence of SFN or DMSO for 24 h. (G) Micrographs of mitochondrial morphology were visualized by MitoTracker Red CMXRos probe staining, and the morphological alterations were quantified by Image J and expressed as mitochondrial interconnectivity. (H) Mitochondrial membrane potential was detected by TMRM staining, and the data was quantified by a flow cytometry and normalized by the average fluorescence density of the Ctrl-shRNA/Man/BSA+DMSO group. (I) The protein expression of mitochondrial fission and fusion related proteins was determined by Western blot, and β-actin was used as a loading control. At least three independent experiments for each study. Data shown in graphs represents the means ± SD. *p < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To examine whether IDH2 is a direct Nrf2 transcriptional target, we analyzed the IDH2 promoter sequence by JASPER (http://jaspar.genreg.net). As shown in Fig. 7C, computational analysis found a putative Nrf2 binding site in the region from −1591 to −1581 that matched the consensus sequence of ARE 5′-RTGAYnnnGCR-3’ [38]. Luciferase reporter analysis demonstrated that knockdown of Nrf2 resulted in decreased IDH2-Full-Luc (−2000-0) activity, whereas the activity of IDH2-short-Luc (−1000-0) and IDH2-mutant-Luc was unaffected (Fig. 7D). In contrast, activating Nrf2 with SFN significantly increased the IDH2-Full-Luc activity, but not the IDH2-Short-Luc and IDH2-mutant-Luc activity (Fig. 7E). In addition, we performed ChIP assay in Healthy-EPCs and confirmed that Nrf2 was directly bound to the sequence “GTGAATTAGCG” of the IDH2 promoter (Fig. 7F). Together, these concordant results demonstrate that Nrf2 upregulates the transcription of IDH2 by directly binding to its promoter.
To confirm whether Nrf2 improving EPC mitochondrial fission induced by diabetes depends on upregulating IDH2 expression, Healthy-EPCs transfected with or without IDH2-shRNA lentivirus were exposed to high glucose and palmitate (HG/PA) to mimic diabetic conditions [39] in the presence or absence of SFN. SFN significantly inhibited mitochondrial fission induced by HG/PA treatment, while knockdown of IDH2 expression with IDH2-shRNA lentivirus almost completely abolished the protective efforts of SFN against HG/PA-induced mitochondrial fission (Fig. 7G). Similarly, the ΔΨm of Healthy-EPCs was decreased when exposed to HG/PA, while SFN treatment increased the ΔΨm of Healthy-EPCs transfected with control shRNA lentivirus but not in Healthy-EPCs transfected with IDH2-shRNA lentivirus (Fig. 7H). Furthermore, we detected the abundance of mitochondrial fusion and fission related proteins, and the results showed that SFN increased Mfn1 and Mfn2 abundance yet decreased Drp1 protein level, while IDH2 knockdown abolished the beneficial action of SFN but without obvious effects on SFN-increased Mfn2 abundance (Fig. 7I). Meanwhile, we evaluated whether IDH2 upregulation could rescue the efforts of knockdown of Nrf2 expression in Healthy-EPCs exposed to HG/PA. As shown in Fig. S6, knockdown of Nrf2 expression exacerbated the mitochondrial damage in Healthy-EPCs exposed to HG/PA, while upregulation of IDH2 expression rescued the mitochondrial damage in Healthy-EPCs induced by knockdown of Nrf2 combined with HG/PA exposure.
Finally, we tested whether the protective roles of Nrf2 in EPC survival and angiogenic function also was dependent on IDH2. SFN treatment significantly protected Healthy-EPCs against HG/PA-induced apoptosis and tube formation impairment; however, after knockdown of IDH2 expression, the protective effects of SFN on Healthy-EPCs exposed to HG/PA were abolished (Figs. S7A and C). In addition, knockdown of Nrf2 expression exacerbated the levels of HG/PA-induced Healthy-EPC apoptosis and tube formation impairment, while upregulation of IDH2 expression counteracted HG/PA-induced apoptosis and tube formation impairment in Healthy-EPCs despite Nrf2 deficiency (Figs. S7B and D). More importantly, IDH2 upregulation efficiently rescued the therapeutic effects of WT-EPCs with Nrf2 deficiency in db/db mice with HLI, which was reflected by significantly rectified blood perfusion recovery (Figs. S8A and B) and angiogenesis (Fig. S8C). These results indicate that Nrf2 improves EPC survival and angiogenic function via upregulation of IDH2 expression under diabetic conditions.
3.7. SFN enhances blood perfusion and angiogenesis in HLI in type 2 diabetic mice
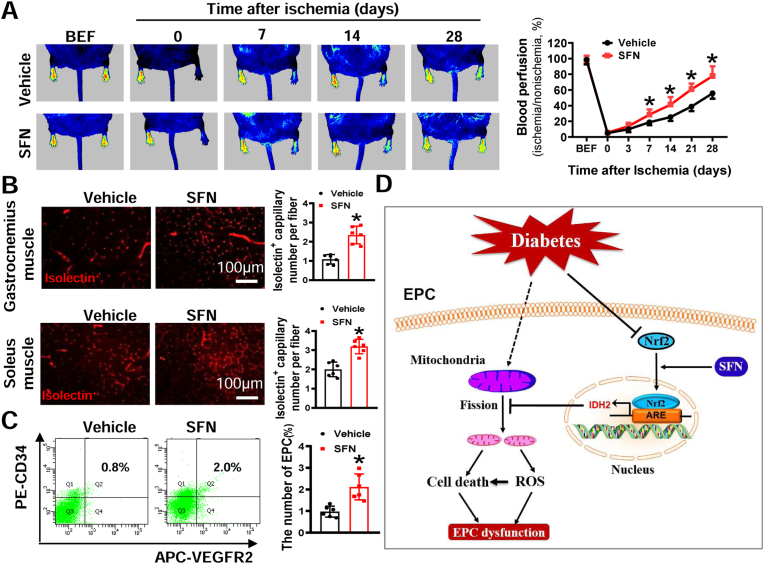
Although upregulation of Nrf2 expression effectively improved the angiogenic function of EPCs under diabetic conditions (Fig. 3), this approach requires infusion of autologous or allogenic EPCs modified by Nrf2 transgene, which has practical limitations in clinical application. If therapeutic efficacy can be achieved using an Nrf2 specific agonist, it will increase the translational value of Nrf2 as a therapeutic target in the treatment of diabetic ischemia vascular diseases. Thus, we evaluated the angiogenic effect of SFN in diabetes. The results showed that SFN treatment improved blood perfusion recovery and promoted angiogenesis in db/db mice with HLI (Fig. 8A and B). Using flow cytometry, we showed that the percentage of circulating EPCs (CD34+/VEGFR2+) was significantly increased in SFN-treated mice compared with controls (Fig. 8C) – a finding consistent with salubrious effects of SFN on EPC survival and function under diabetic conditions.
Fig. 8.
SFN enhances blood perfusion and angiogenesis in db/db mice with HLI.db/db mice (FVB background) were pretreated with SFN for 1 week, followed by HLI surgery and continual SFN treatment for additional 4 weeks. (A) The time-course of blood perfusion before (BEF) and after HLI surgery was monitored by a Pericam Perfusion Speckle Imager, and the data was quantified by Image J and expressed as the percentage of the perfusion relative to the collateral nonischemic limb. (B) Transverse sections of ischemic gastrocnemius and soleus muscle were stained with Alexa Fluor® 594 conjugated isolectin to enumerate isolectin-stained cell number as a proxy of capillary density. Capillary density was expressed as isolectin+ capillaries per muscle fiber. (C) At day 7 after HLI surgery, peripheral blood was collected to evaluate the percentage of EPCs (CD34+/VEGFR2+) in circulation by a flow cytometry. n = 6 mice per group. Data shown in graphs represent the means ± SD. *P < 0.05 vs Vehicle group. (D) Schematic illustration of the protective effects of Nrf2 on EPCs under diabetic conditions. Diabetes decreases expression of Nrf2 in EPCs and induces mitochondrial fission and dysfunction, resulting in reactive oxygen species (ROS) overproduction and cell death, ultimately leading to EPC dysfunction. Upregulation of Nrf2 by transgene or supplementation of SFN can rescue diabetes-induced inhibition of Nrf2 transcriptional activity, resulting in upregulation of IDH2 transcription, which inhibits mitochondrial fission, rectifies mitochondrial function, and decreases ROS production and cell death, ultimately rescues EPC dysfunction. ARE, antioxidant responsive element.
4. Discussion
Endothelial dysfunction is a critical pathophysiological change that contributes to diabetic vascular complications. EPCs play an important role in endothelial repair and angiogenesis [17]. Thus, improving diabetic EPC function is crucial to improve diabetes-induced endothelial dysfunction and to limit progression of vascular complications. Our previous study indicates that Nrf2 may be a target for improving diabetic EPC function [3]. In the present study, we further clarify Nrf2's mechanistic action in orchestrating EPC functions. First, we find a novel relationship between Nrf2 activity and EPC dysfunction in both diabetic patients and diabetic mice demonstrating that Nrf2 could be a therapeutic target with translational potential via Nrf2 activators such as SFN. Second, we provide solid evidence that Nrf2 rescue of impaired EPC function in diabetes is tightly coupled with fine tuning of mitochondrial dynamics. Our third insight is that IDH2 likely contributes to the beneficial effects of Nrf2 in diabetic EPCs by coordinating mitochondrial homeostasis and function.
Our finding of the strong relationship between Nrf2 activity and EPC dysfunction in both diabetic patients and diabetic mice is consistent with Nrf2 as an essential redox and metabolic regulator of stem and progenitor cell state and function [17]. Moreover, impaired EPC function in diabetic patients likely is associated with the suppression of Nrf2 and its downstream genes [40]. Additional, preclinical studies show that knockout of Nrf2 attenuates survival, proliferation, migration, and angiogenic function of mouse proangiogenic cells [41]; and silencing of Nrf2 by shRNA impairs the angiogenic function of mouse EPC [42,43] or human cord blood endothelial colony-forming cell (ECFC) [44] and inhibits secretion of proangiogenic factors. In our present study, we find that the expression levels of Nrf2 and its downstream genes are significantly lower in EPCs from both diabetic patients and diabetic mice, which are associated with remarkable impairment of the cell survival and angiogenic function (Fig. 1 and Fig. S1). These observations indicate not only a strong relationship between Nrf2 activity and diabetic EPC dysfunction, but also the biological consistency of Nrf2 and EPC function across species. Moreover, we reveal that elevating Nrf2 expression in EPCs from both diabetic patients and diabetic mice rescues the survival and angiogenic capacity, and promotes mouse diabetic EPCs-mediated angiogenesis and blood perfusion recovery in a db/db HLI model; while silencing of Nrf2 expression in normal EPCs from both healthy donors and WT mice weakens EPC survival and angiogenic capacity, and impairs mouse WT-EPCs-mediated angiogenesis and blood perfusion recovery in a db/db HLI model (Fig. 2, Fig. S2, and Fig. 3). Collectively, these results reflect a decline in Nrf2 levels is a significant, causative factor in development of diabetic EPC dysfunction, although the mechanism underlying this process remains to be understood. Furthermore, we find that ‘Nrf2 rescue’ is a promising therapeutic approach for improving the reparative capacity of EPCs in patients with diabetes.
We show that Nrf2 activator, SFN, pretreatment of diabetic mice improves blood perfusion recovery and angiogenesis in db/db mice with HLI – an effect accompanied by a significant increase of EPCs in the peripheral blood (Fig. 8). This is consistent with SFN efficacy in prevention of diabetes-induced cardiac dysfunction via activation of Nrf2 in both streptozotocin-induced diabetic mice and db/db diabetic mice [33,45]. Thus, SFN pretreatment improves EPC number and angiogenic function likely via activation of Nrf2 in EPCs. Indeed, emerging evidence demonstrates that preconditioning of EPCs with pharmacologic activators of Nrf2 protects EPC function. For example, preincubation of human ECFCs with SFN reduces intracellular ROS in the presence of H2O2 and preserves ECFC migration and tube formation [44], and preconditioning of diabetic mouse EPCs with a different Nrf2 activator, tert-butylhydroquinone, protects EPC functions from oxidative stress and senescence via increasing antioxidant gene expression and reducing ROS [43]. Combining these ex vivo studies with our in vivo findings provide support that preconditioning EPCs with Nrf2 inducers is a viable strategy to protect EPCs against stress conditions, and thus, support Nrf2 induction is a promising therapeutic approach to treat diabetic ischemia vascular diseases, and Nrf2 inducers will need systemic preclinical and/or clinical validation.
It is worth noting that global deletion of Nrf2 also augments blood perfusion recovery and angiogenesis in non-diabetic mice with HLI [41,46]. This is thought to be resulted from impaired antioxidant defense and increased accumulation of ROS in endothelial cells and skeletal muscle tissues because of global Nrf2 deficiency; consequently, enhanced inflammatory response and promoted inflammatory cell infiltration in the ischemic muscle tissues facilitate ischemic angiogenesis through paracrine mechanisms in healthy mice [41,46]. However, whether Nrf2 deficiency also benefits ischemic angiogenesis in diabetic mice that associated with excessive systemic inflammation remains uncertain, which needs to be further investigated in future studies.
Nrf2-mediated rescue of diabetic EPC functions is strongly linked with beneficial changes in EPC mitochondrial dynamics. Although this is a novel finding, accumulating evidence demonstrates that mitochondrial dysfunctions are strongly associated with diabetic complications [47]. Moreover, mitochondrial dysfunction results in overproduction of ROS and low levels of ATP generation that contribute to cellular dysfunction. Of course, mitochondrial function depends on quality control and dynamics via fusion and fission process in response to various stresses [22,37], and previous studies show that altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes [48]. However, it remains largely unknown how important mitochondrial dynamics are for EPC function and whether Nrf2 protects diabetic EPC function by regulating mitochondrial dynamics. In the present study, we find that diabetic EPC dysfunction and associated Nrf2 dysregulation are accompanied by obvious mitochondrial fragmentation and dysfunction reflected by increased intracellular ROS and mtROS production and decreased ΔΨm and ATP generation (Fig. 4). Importantly, shRNA-mediated knockdown of Nrf2 in healthy EPCs mimics diabetes-induced phenotype, while upregulation of Nrf2 expression in diabetic EPCs reverses diabetes-induced mitochondrial fragmentation and dysfunction (Fig. 5). We also find that mitochondrial fission inhibitor (Mdivil-1) rescues the effects of Nrf2 silencing on healthy EPC survival and tube formation ability, whereas mitochondrial fission activator (FCCP) nearly abolishes the protective effects of elevated Nrf2 expression on diabetic EPC survival and tube formation capacity (Fig. S3). Therefore, we conclude that Nrf2-medaited rescue of diabetic EPC function is highly dependent on finely tuning mitochondrial dynamics to correct mitochondrial dysfunction.
Mitochondrial dynamics are regulated by the activity of the dynamin superfamily of GTPases, including fusion regulating proteins (Mfn1, Mfn2 and Opa1) and fission regulating proteins (Drp1 and Fis1) [24]. An imbalance between mitochondrial fission and fusion regulatory proteins contributes to diabetic complications, and thus, targeting mitochondrial regulatory proteins to protect against diabetes-induced multi-organ injury is widely appreciated [49,50]. A previous study has linked Nrf2 activation to mitochondrial hyperfusion in tumor cells [51]. However, whether Nrf2 inhibiting mitochondrial fission in diabetic EPCs through directly transcriptional regulating mitochondrial dynamic proteins remains unclear. We demonstrate here that diabetic EPC mitochondrial fragmentation and Nrf2 dysregulation are accompanied by remarkable upregulation of mitochondrial fission proteins (i.e.: Drp1 and Fis1) and downregulation of fusion proteins (i.e.: Opa1, Mfn1 and Mfn2) (Fig. 4) – conditions recaptured by shRNA-mediated knockdown of Nrf2 in healthy EPCs and reversed by lentivirus-mediated upregulation of Nrf2 in diabetic EPCs (Fig. 6). Notably, neither knockdown of Nrf2 expression in healthy EPCs nor upregulating Nrf2 expression in diabetic EPCs significantly affects mRNA expression of fission and fusion regulatory proteins (Fig. 6), implying that mitochondrial fission and fusion regulatory proteins are not direct downstream transcriptional targets of Nrf2 for regulating EPC mitochondrial dynamics.
Here in our RNA-seq assay screen, among genes positively affected by Nrf2 manipulation, only IDH2 relates directly to mitochondrial function, indicating that IDH2 may be a potential target gene of Nrf2 to regulate mitochondrial dynamics in EPCs (Fig. S4). In fact, our luciferase reporter analysis and Chip assay demonstrate that Nrf2 directly binds with IDH2 promoter and regulates its transcription in EPCs (Fig. 7C–F). Thus, IDH2 is a direct downstream transcriptional target gene of Nrf2 and likely plays an essential role in Nrf2-nedauted regulation of mitochondrial dynamics and function in EPCs. IDH2 is a mitochondrial NADP(+)-dependent isocitrate dehydrogenase that catalyzes the oxidative decarboxylation of isocitrate to α-ketoglutarate [52], thereby producing NADPH, which is not only essential for the regeneration of the thioredoxin system, but also for keeping the GSH-pool in a reduced state [53]. Therefore, IDH2 plays an essential role in controlling mitochondrial redox homeostasis and attenuating oxidative stress damage. Notably, IDH2 deficiency causes mitochondrial dysfunction and disrupts redox homeostasis in cardiomyocytes and cancer cells [54,55], and loss of IDH2 leads to endothelial dysfunction through the induction of mitochondrial dynamic changes [56]. In addition, IDH2 can reprogram mitochondrial dynamics in cancer cells, wherein loss of IDH2 significantly increases mitochondrial fission [57]. Consistent with all these findings, our study demonstrates that lentivirus-mediated shRNA knockdown of IDH2 almost completely abrogates the beneficial effects of Nrf2 activation by SFN on mitochondrial dynamics, ΔΨm, ATP generation, and fission and fusion protein expression in healthy EPCs exposed to HG/PA (Fig. 7G–I), while the forced overexpression of IDH2 gene rescues the mitochondrial dysfunction in human healthy EPCs due to Nrf2 knockdown and HG/PA exposure in vitro (Fig. S6) and rescues the therapeutic effects of mouse WT-EPCs with Nrf2 deficiency in db/db mice with HLI (Fig. S8). Thus, we demonstrate for the first time that IDH2 is a direct downstream transcriptional target gene of Nrf2 and plays essential roles in Nrf2 regulating mitochondrial dynamic and function in EPCs.
In conclusion, upregulation of Nrf2 improves EPC survival and function under diabetic conditions. This is because of improved mitochondrial dynamics and function in diabetic EPCs, which is likely mediated by Nrf2 transcriptional upregulation of IDH2 expression as illustrated in Fig. 8D. Pharmacological activation of Nrf2 by supplementation of Nrf2 inducer such as SFN can enhance diabetic ischemia angiogenesis and blood perfusion recovery, highlighting a clinical relevance for diabetic vascular ischemia treatment.
Author contributions
X.D., X.Y., Y.T. conceived and designed the study. X.D., K.W., J.F., X.F., Q.L., Y.C., H.L., H.C., Y.L., and O.C. performed in vitro cell culture, cell biology, molecular biology experiments, and histology analysis. X.D., K.W., and J.C. performed in vivo therapeutic studies in mice. X.D., K.W., J.F., X.F., X.L., D.R., J.L. and Y.T. analyzed the data. Y.L., and L.C. provided critical materials. X.D. and Y.T. wrote the manuscript. D.C., K.W., J.L., and L.C. edited the manuscript with important intellectual content. X.D., X.Y., and Y.T. supervised this study.
Declaration of competing interest
No potential conflicts of interest relevant to this article were reported.
Acknowledgments
This study was supported in part by the National Natural Science Foundation of China (82170420, 81770305, 81873466), National Key R&D Program of China (2017YFA0506000), Junior Faculty Award (1-13-JF-53) from American Diabetes Association, Disciplinary Construction Innovation Team Foundation of Chengdu Medical College (CMC-XK-2101), Natural Science Foundation of Zhejiang Province (LY22H070005, LY22H020005), Grant from Science and Technology Bureau of Wenzhou (Y20210010), and Basic Scientific Research Foundation of Wenzhou Medical University (KYYW201907).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2022.102449.
Contributor Information
Xiaoqing Yan, Email: yanxiaoqing1128@163.com.
Yi Tan, Email: yi.tan@louisville.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Jarajapu Y.P., Grant M.B. The promise of cell-based therapies for diabetic complications: challenges and solutions. Circ. Res. 2010;106:854–869. doi: 10.1161/CIRCRESAHA.109.213140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fadini G.P., Losordo D., Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ. Res. 2012;110:624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai X., Yan X., Zeng J., Chen J., Wang Y., Chen J., Li Y., Barati M.T., Wintergerst K.A., Pan K., Nystoriak M.A., Conklin D.J., Rokosh G., Epstein P.N., Li X., Tan Y. Elevating cxcr7 improves angiogenic function of epcs via akt/gsk-3beta/fyn-mediated nrf2 activation in diabetic limb ischemia. Circ. Res. 2017;120:e7–e23. doi: 10.1161/CIRCRESAHA.117.310619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan X., Dai X., He L., Ling X., Shao M., Zhang C., Wang Y., Xiao J., Cai L., Li X., Tan Y. A novel cxcr4 antagonist enhances angiogenesis via modifying the ischaemic tissue environment. J. Cell Mol. Med. 2017;21:2298–2307. doi: 10.1111/jcmm.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler J.M., Nolan D.J., Vertes E.L., Varnum-Finney B., Kobayashi H., Hooper A.T., Seandel M., Shido K., White I.A., Kobayashi M., Witte L., May C., Shawber C., Kimura Y., Kitajewski J., Rosenwaks Z., Bernstein I.D., Rafii S. Endothelial cells are essential for the self-renewal and repopulation of notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murohara T., Ikeda H., Duan J., Shintani S., Sasaki K., Eguchi H., Onitsuka I., Matsui K., Imaizumi T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J. Clin. Invest. 2000;105:1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamihata H., Matsubara H., Nishiue T., Fujiyama S., Amano K., Iba O., Imada T., Iwasaka T. Improvement of collateral perfusion and regional function by implantation of peripheral blood mononuclear cells into ischemic hibernating myocardium. Arterioscler. Thromb. Vasc. Biol. 2002;22:1804–1810. doi: 10.1161/01.atv.0000039168.95670.b9. [DOI] [PubMed] [Google Scholar]
- 8.Loomans C.J.M., de Koning E.J.P., Staal F.J.T., Rookmaaker M.B., Verseyden C., de Boer H.C., Verhaar M.C., Braam B., Rabelink T.J., van Zonneveld A.J. Endothelial progenitor cell dysfunction - a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 9.Fadini G.P., Boscaro E., Albiero M., Menegazzo L., Frison V., de Kreutzenberg S., Agostini C., Tiengo A., Avogaro A. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes possible role of stromal-derived factor-1 alpha. Diabetes Care. 2010;33:1607–1609. doi: 10.2337/dc10-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim K.A., Shin Y.J., Kim J.H., Lee H., Noh S.Y., Jang S.H., Bae O.N. Dysfunction of endothelial progenitor cells under diabetic conditions and its underlying mechanisms. Arch Pharm. Res. (Seoul) 2012;35:223–234. doi: 10.1007/s12272-012-0203-y. [DOI] [PubMed] [Google Scholar]
- 11.Callaghan M.J., Ceradini D.J., Gurtner G.C. Hyperglycemia-induced reactive oxygen species and impaired endothelial progenitor cell function. Antioxidants Redox Signal. 2005;7:1476–1482. doi: 10.1089/ars.2005.7.1476. [DOI] [PubMed] [Google Scholar]
- 12.Hamed S., Brenner B., Abassi Z., Aharon A., Daoud D., Roguin A. Hyperglycemia and oxidized-ldl exert a deleterious effect on endothelial progenitor cell migration in type 2 diabetes mellitus. Thromb. Res. 2010;126:166–174. doi: 10.1016/j.thromres.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Yu J.W., Deng Y.P., Han X., Ren G.F., Cai J., Jiang G.J. Metformin improves the angiogenic functions of endothelial progenitor cells via activating ampk/enos pathway in diabetic mice. Cardiovasc. Diabetol. 2016;15:88. doi: 10.1186/s12933-016-0408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jialal I., Fadini G.P., Pollock K., Devaraj S. Circulating levels of endothelial progenitor cell mobilizing factors in the metabolic syndrome. Am. J. Cardiol. 2010;106:1606–1608. doi: 10.1016/j.amjcard.2010.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desouza C.V., Hamel F.G., Bidasee K., O'Connell K. Role of inflammation and insulin resistance in endothelial progenitor cell dysfunction. Diabetes. 2011;60:1286–1294. doi: 10.2337/db10-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wils J., Favre J., Bellien J. Modulating putative endothelial progenitor cells for the treatment of endothelial dysfunction and cardiovascular complications in diabetes. Pharmacol. Ther. 2017;170:98–115. doi: 10.1016/j.pharmthera.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Dai X., Yan X., Wintergerst K.A., Cai L., Keller B.B., Tan Y. Nrf2: redox and metabolic regulator of stem cell state and function. Trends Mol. Med. 2020;26:185–200. doi: 10.1016/j.molmed.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Al-Sawaf O., Clarner T., Fragoulis A., Kan Y.W., Pufe T., Streetz K., Wruck C.J. Nrf2 in health and disease: current and future clinical implications. Clin. Sci. (Lond.) 2015;129:989–999. doi: 10.1042/CS20150436. [DOI] [PubMed] [Google Scholar]
- 19.Yu B.B., Zhi H., Zhang X.Y., Liang J.W., He J., Su C., Xia W.H., Zhang G.X., Tao J. Mitochondrial dysfunction-mediated decline in angiogenic capacity of endothelial progenitor cells is associated with capillary rarefaction in patients with hypertension via downregulation of cxcr4/jak2/sirt5 signaling. EBioMedicine. 2019;42:64–75. doi: 10.1016/j.ebiom.2019.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen G.X. Oxidative stress and diabetic cardiovascular disorders: roles of mitochondria and nadph oxidase. Can. J. Physiol. Pharmacol. 2010;88:241–248. doi: 10.1139/Y10-018. [DOI] [PubMed] [Google Scholar]
- 21.Sorrentino S.A., Bahlmann F.H., Besler C., Muller M., Schulz S., Kirchhoff N., Doerries C., Horvath T., Limbourg A., Limbourg F., Fliser D., Haller H., Drexler H., Landmesser U. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2007;116:163–173. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- 22.Ni H.M., Williams J.A., Ding W.X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015;4:6–13. doi: 10.1016/j.redox.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra P., Chan D.C. Metabolic regulation of mitochondrial dynamics. J. Cell Biol. 2016;212:379–387. doi: 10.1083/jcb.201511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rovira-Llopis S., Banuls C., Diaz-Morales N., Hernandez-Mijares A., Rocha M., Victor V.M. Mitochondrial dynamics in type 2 diabetes: pathophysiological implications. Redox Biol. 2017;11:637–645. doi: 10.1016/j.redox.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding M., Feng N., Tang D., Feng J., Li Z., Jia M., Liu Z., Gu X., Wang Y., Fu F., Pei J. Melatonin prevents drp1-mediated mitochondrial fission in diabetic hearts through sirt1-pgc1alpha pathway. J. Pineal Res. 2018;65 doi: 10.1111/jpi.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang T.C. Nuclear factor-erythroid 2-related factor 2 (nrf2) and mitochondrial dynamics/mitophagy in neurological diseases. Antioxidants. 2020;9:617. doi: 10.3390/antiox9070617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang K., Dai X., He J., Yan X., Yang C., Fan X., Sun S., Chen J., Xu J., Deng Z., Fan J., Yuan X., Liu H., Carlson E.C., Shen F., Wintergerst K.A., Conklin D.J., Epstein P.N., Lu C., Tan Y. Endothelial overexpression of metallothionein prevents diabetes-induced impairment in ischemia angiogenesis through preservation of hif-1alpha/sdf-1/vegf signaling in endothelial progenitor cells. Diabetes. 2020;69:1779–1792. doi: 10.2337/db19-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan X., Cai S., Xiong X., Sun W., Dai X., Chen S., Ye Q., Song Z., Jiang Q., Xu Z. Chemokine receptor cxcr7 mediates human endothelial progenitor cells survival, angiogenesis, but not proliferation. J. Cell. Biochem. 2012;113:1437–1446. doi: 10.1002/jcb.24015. [DOI] [PubMed] [Google Scholar]
- 29.Dai X., Tan Y., Cai S., Xiong X., Wang L., Ye Q., Yan X., Ma K., Cai L. The role of cxcr7 on the adhesion, proliferation and angiogenesis of endothelial progenitor cells. J. Cell Mol. Med. 2011;15:1299–1309. doi: 10.1111/j.1582-4934.2011.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koopman W.J., Verkaart S., Visch H.J., van der Westhuizen F.H., Murphy M.P., van den Heuvel L.W., Smeitink J.A., Willems P.H. Inhibition of complex i of the electron transport chain causes o2-. -mediated mitochondrial outgrowth. Am. J. Physiol. Cell Physiol. 2005;288:C1440–C1450. doi: 10.1152/ajpcell.00607.2004. [DOI] [PubMed] [Google Scholar]
- 31.Schmitt K., Grimm A., Dallmann R., Oettinghaus B., Restelli L.M., Witzig M., Ishihara N., Mihara K., Ripperger J.A., Albrecht U., Frank S., Brown S.A., Eckert A. Circadian control of drp1 activity regulates mitochondrial dynamics and bioenergetics. Cell Metabol. 2018;27:657–666. doi: 10.1016/j.cmet.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Chen H., Yuan R., Zhang Y., Zhang X., Chen L., Zhou X., Yuan Z., Nie Y., Li M., Mo D., Chen Y. Atf4 regulates srebp1c expression to control fatty acids synthesis in 3t3-l1 adipocytes differentiation. Biochim. Biophys. Acta. 2016;1859:1459–1469. doi: 10.1016/j.bbagrm.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Xu Z., Wang S., Ji H., Zhang Z., Chen J., Tan Y., Wintergerst K., Zheng Y., Sun J., Cai L. Broccoli sprout extract prevents diabetic cardiomyopathy via nrf2 activation in db/db t2dm mice. Sci. Rep. 2016;6 doi: 10.1038/srep30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xin Y., Bai Y., Jiang X., Zhou S., Wang Y., Wintergerst K.A., Cui T., Ji H., Tan Y., Cai L. Sulforaphane prevents angiotensin ii-induced cardiomyopathy by activation of nrf2 via stimulating the akt/gsk-3ss/fyn pathway. Redox Biol. 2018;15:405–417. doi: 10.1016/j.redox.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishikawa T., Edelstein D., Du X.L., Yamagishi S., Matsumura T., Kaneda Y., Yorek M.A., Beebe D., Oates P.J., Hammes H.P., Giardino I., Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 36.Yu T., Jhun B.S., Yoon Y. High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxidants Redox Signal. 2011;14:425–437. doi: 10.1089/ars.2010.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan D.C. Mitochondrial dynamics and its involvement in disease. Annu. Rev. Pathol. 2020;15:235–259. doi: 10.1146/annurev-pathmechdis-012419-032711. [DOI] [PubMed] [Google Scholar]
- 38.Rushmore T.H., Morton M.R., Pickett C.B. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- 39.Liu N., Wu J., Zhang L., Gao Z., Sun Y., Yu M., Zhao Y., Dong S., Lu F., Zhang W. Hydrogen sulphide modulating mitochondrial morphology to promote mitophagy in endothelial cells under high-glucose and high-palmitate. J. Cell Mol. Med. 2017;21:3190–3203. doi: 10.1111/jcmm.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Issan Y., Hochhauser E., Kornowski R., Leshem-Lev D., Lev E., Sharoni R., Vanella L., Puri N., Laniado-Schwartzman M., Abraham N.G., Porat E. Endothelial progenitor cell function inversely correlates with long-term glucose control in diabetic patients: Association with the attenuation of the heme oxygenase-adiponectin axis. Can. J. Cardiol. 2012;28:728–736. doi: 10.1016/j.cjca.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Florczyk U., Jazwa A., Maleszewska M., Mendel M., Szade K., Kozakowska M., Grochot-Przeczek A., Viscardi M., Czauderna S., Bukowska-Strakova K., Kotlinowski J., Jozkowicz A., Loboda A., Dulak J. Nrf2 regulates angiogenesis: effect on endothelial cells, bone marrow-derived proangiogenic cells and hind limb ischemia. Antioxidants Redox Signal. 2014;20:1693–1708. doi: 10.1089/ars.2013.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang R., Liu L., Liu H., Wu K., Liu Y., Bai L., Wang Q., Qi B., Qi B., Zhang L. Reduced nrf2 expression suppresses endothelial progenitor cell function and induces senescence during aging. Aging (Albany NY) 2019;11:7021–7035. doi: 10.18632/aging.102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang R.Y., Liu L.H., Liu H., Wu K.F., An J., Wang Q., Liu Y., Bai L.J., Qi B.M., Qi B.L., Zhang L. Nrf2 protects against diabetic dysfunction of endothelial progenitor cells via regulating cell senescence. Int. J. Mol. Med. 2018;42:1327–1340. doi: 10.3892/ijmm.2018.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gremmels H., de Jong O.G., Hazenbrink D.H., Fledderus J.O., Verhaar M.C. The transcription factor nrf2 protects angiogenic capacity of endothelial colony-forming cells in high-oxygen radical stress conditions. Stem Cell. Int. 2017;2017 doi: 10.1155/2017/4680612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gu J., Cheng Y., Wu H., Kong L., Wang S., Xu Z., Zhang Z., Tan Y., Keller B.B., Zhou H., Wang Y., Xu Z., Cai L. Metallothionein is downstream of nrf2 and partially mediates sulforaphane prevention of diabetic cardiomyopathy. Diabetes. 2017;66:529–542. doi: 10.2337/db15-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ichihara S., Yamada Y., Liu F., Murohara T., Itoh K., Yamamoto M., Ichihara G. Ablation of the transcription factor nrf2 promotes ischemia-induced neovascularization by enhancing the inflammatory response. Arterioscler. Thromb. Vasc. Biol. 2010;30:1553–1561. doi: 10.1161/ATVBAHA.110.204123. [DOI] [PubMed] [Google Scholar]
- 47.Kim J.A., Wei Y., Sowers J.R. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008;102:401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shenouda S.M., Widlansky M.E., Chen K., Xu G., Holbrook M., Tabit C.E., Hamburg N.M., Frame A.A., Caiano T.L., Kluge M.A., Duess M.A., Levit A., Kim B., Hartman M.L., Joseph L., Shirihai O.S., Vita J.A. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124:444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu L., Ding M., Tang D., Gao E., Li C., Wang K., Qi B., Qiu J., Zhao H., Chang P., Fu F., Li Y. Targeting mitochondrial dynamics by regulating mfn2 for therapeutic intervention in diabetic cardiomyopathy. Theranostics. 2019;9:3687–3706. doi: 10.7150/thno.33684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu Q., Zhang H., Gutierrez Cortes N., Wu D., Wang P., Zhang J., Mattison J.A., Smith E., Bettcher L.F., Wang M., Lakatta E.G., Sheu S.S., Wang W. Increased drp1 acetylation by lipid overload induces cardiomyocyte death and heart dysfunction. Circ. Res. 2020;126:456–470. doi: 10.1161/CIRCRESAHA.119.315252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabouny R., Fraunberger E., Geoffrion M., Ng A.C., Baird S.D., Screaton R.A., Milne R., McBride H.M., Shutt T.E. The keap1-nrf2 stress response pathway promotes mitochondrial hyperfusion through degradation of the mitochondrial fission protein drp1. Antioxidants Redox Signal. 2017;27:1447–1459. doi: 10.1089/ars.2016.6855. [DOI] [PubMed] [Google Scholar]
- 52.Reitman Z.J., Yan H. Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism. J. Natl. Cancer Inst. 2010;102:932–941. doi: 10.1093/jnci/djq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller C.G., Holmgren A., Arner E.S.J., Schmidt E.E. Nadph-dependent and -independent disulfide reductase systems. Free Radic. Biol. Med. 2018;127:248–261. doi: 10.1016/j.freeradbiomed.2018.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Das A., Durrant D., Salloum F.N., Xi L., Kukreja R.C. Pde5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol. Ther. 2015;147:12–21. doi: 10.1016/j.pharmthera.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen X., Zhuo S., Xu W., Chen X., Huang D., Sun X., Cheng Y. Isocitrate dehydrogenase 2 contributes to radiation resistance of oesophageal squamous cell carcinoma via regulating mitochondrial function and ros/pakt signalling. Br. J. Cancer. 2020;123:126–136. doi: 10.1038/s41416-020-0852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park J.B., Nagar H., Choi S., Jung S.B., Kim H.W., Kang S.K., Lee J.W., Lee J.H., Park J.W., Irani K., Jeon B.H., Song H.J., Kim C.S. Idh2 deficiency impairs mitochondrial function in endothelial cells and endothelium-dependent vasomotor function. Free Radic. Biol. Med. 2016;94:36–46. doi: 10.1016/j.freeradbiomed.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y., Agarwal E., Bertolini I., Ghosh J.C., Seo J.H., Altieri D.C. Idh2 reprograms mitochondrial dynamics in cancer through a hif-1alpha-regulated pseudohypoxic state. Faseb. J. 2019;33:13398–13411. doi: 10.1096/fj.201901366R. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.