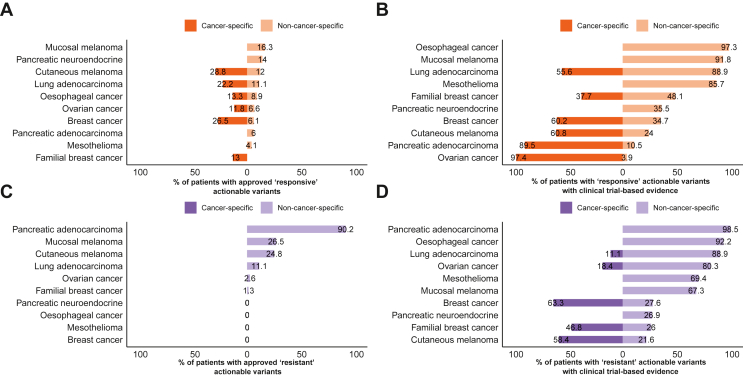
Figure 2.
Repurposing potential of datasets analysed. Percentage of patients with cancer-specific and non-cancer-specific Food and Drug Administration (FDA)-approved (A) sensitive and (C) resistance biomarkers. Percentage of patients with (B) sensitive and (D) resistance biomarkers for drugs in clinical trials. Bars to the left of x = 0 indicate percentage of patients who can be prescribed an on-label drug, bars to the right of x = 0 indicate percentage of patients who could be prescribed an off-label drug. Off-label prescriptions are additive to the on-label prescriptions.