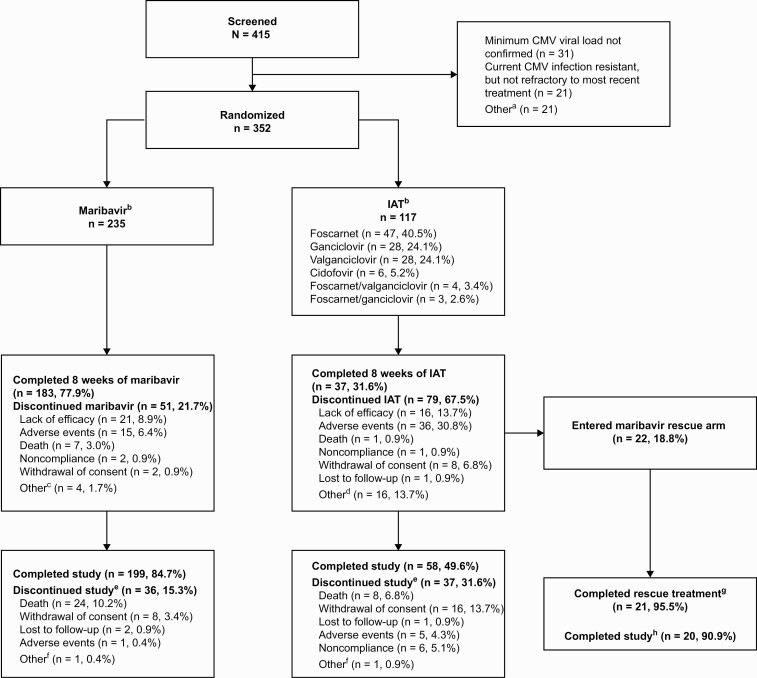
Figure 2.
Patient disposition at enrollment, randomization, and follow-up. Percentages were calculated based on the number of patients randomized to each treatment group. Percentages may not total to 100% due to rounding. Serious AEs were recorded until the end of trial participation or resolution (whichever was later); median on-study duration was 141.0 days in each group. aPatients could have multiple reasons for not being randomized. Other reasons were: patient did not receive an HCT or SOT (n = 1); CMV infection not confirmed refractory to most recent treatment (n = 2); investigator not willing to treat the patient with ganciclovir, valganciclovir, foscarnet, or cidofovir (n = 2); platelet count <25 000/mm3 (n = 5); hemoglobin <8 g/dL (n = 1); eGFR ≤30 mL/min/1.73 m2 (n = 1); pregnancy (n = 1); patient was not willing/not able to comply fully with study procedures/restrictions (n = 3); current refractory or resistant CMV infection due to inadequate adherence to prior treatment (n = 2); serum aspartate aminotransferase >5 × ULN at screening, or serum alanine aminotransferase >5 × ULN at screening, or total bilirubin ≥3.0 × ULN at screening (n = 1); received any investigational agent with known anti-CMV activity within 30 days before initiation of study treatment or investigational CMV vaccine at any time (n = 1); and active malignancy (n = 1). bOne patient per group was randomized but did not receive trial medication. Percentage for each IAT type was calculated based on n = 116. cOther reasons for treatment discontinuation in the maribavir group included investigator decision to switch to letermovir, CMV detected in patient’s cerebrospinal fluid, nothing-by-mouth status with mental status change with risk for aspiration, and disease progression (in 1 patient each). dOther reasons for treatment discontinuation in the IAT group were: low viral load/CMV clearance (with concern of toxicity with continued administration of IAT (n = 9), patient safety (n = 3), patient/investigator request (n = 2), no efficacy and patient ineligible for rescue therapy (n = 1), and peripherally inserted central catheter issues (n = 1). eThese results are based on investigator determination for the primary reason for study discontinuation. fOther reasons for study discontinuation in maribavir or IAT group included investigator discretion to discontinue 1 patient before dosing with maribavir, and no efficacy with IAT for a patient who was not eligible for rescue therapy. gPer protocol, maribavir rescue arm treatment was discontinued in 1 patient due to CMV encephalitis. hOne patient was unable to complete follow-up visits in the study due to hospitalization in a different city and therefore did not complete the maribavir rescue study period. Abbreviations: AE, adverse event; CMV, cytomegalovirus; eGFR, estimated glomerular filtration rate; HCT, hematopoietic-cell transplant; IAT, investigator-assigned therapy; SOT, solid-organ transplant; ULN, upper limit of normal.