Figure 2.
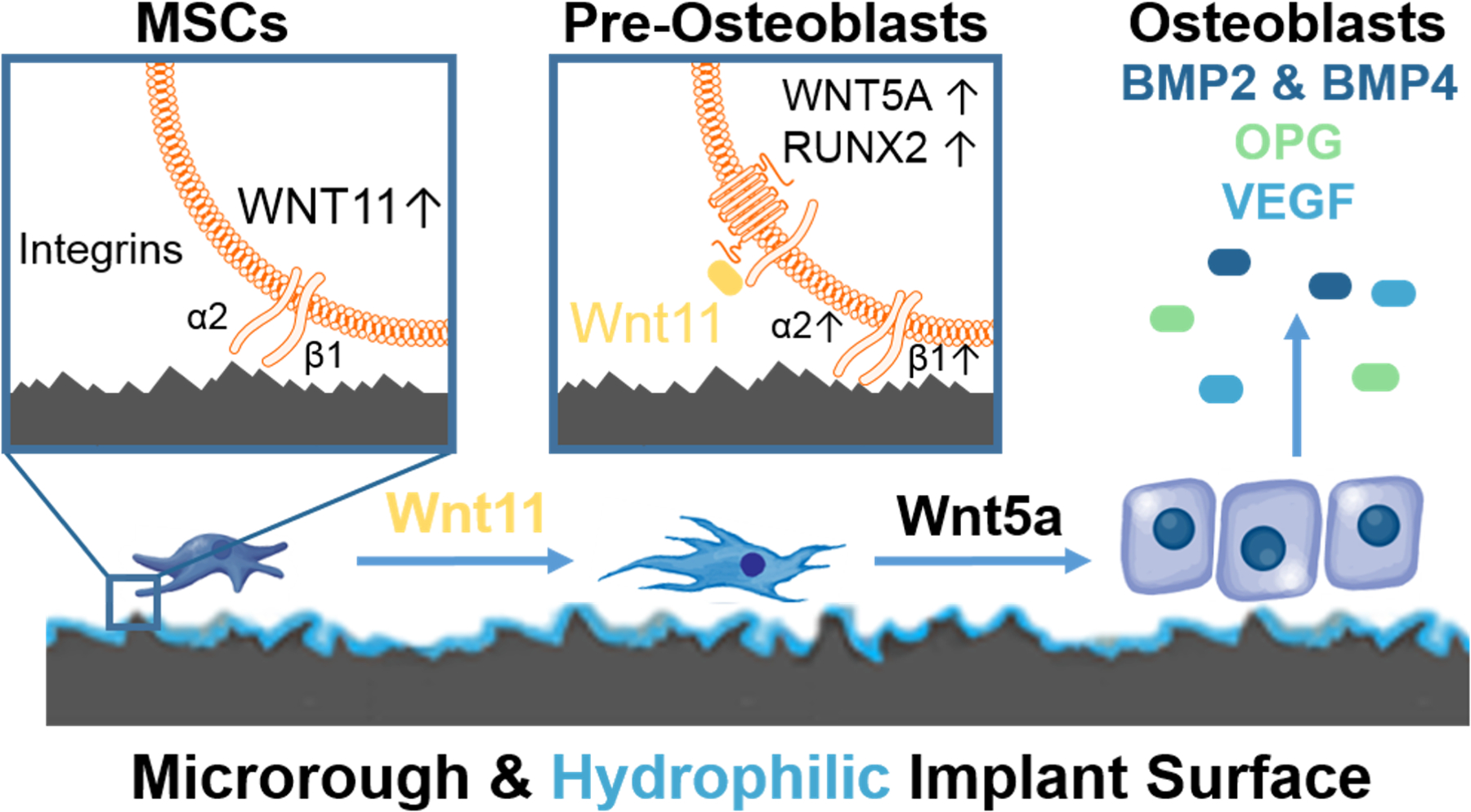
Mechanisms involved in the regulation of osteoblastic differentiation of bone marrow stromal cells (MSCs) on microstructured Ti-based implant surfaces. MSCs migrate to the implant environment and attach via α5β1 integrin binding to fibronectin. MSCs begin to sense the implant architecture shifting to production of α2β1 integrin binding to collagen type 1 and upregulate non-canonical Wnt11 (left panel). Wnt11 acts internally and externally of the cell to increase the number of pre-osteoblasts in the implant environment. This method is achieved by increasing production Wnt5a, and RUNX2 transcription in the nucleus (middle panel). These pre-osteoblasts mature into osteoblasts that product robust concentrations of bone morphogenetic protein 2 and 4, osteoprotegerin (OPG), and vascular endothelial growth factor (VEGF) necessary for bone apposition and mineralization (right panel).
