Abstract
Introduction:
Splanchnic vein thrombosis (SVT) occurs in a heterogenous group of patients secondary to a variety of risk factors including liver disease. Minimal data regarding natural history and outcomes of SVT exists to inform management decisions. As such, there is equipoise regarding the utility of anticoagulation in cirrhotic patients with SVT. We sought to identify clinical factors predictive of new or progressive thrombosis in a cohort of patients with untreated SVT.
Methods:
We conducted a retrospective cohort study of cirrhotic patients over 18 years of age diagnosed with SVT at the Oregon Health & Science University from 2015 to 2020, excluding those initially treated with anticoagulation. The primary study endpoint was a composite of the following: imaging-confirmed progression of SVT, development of cavernous transformation, intestinal ischemia, portal cholangiopathy or new venous or arterial thrombosis.
Results:
261 patients were included in the analysis (median age 61 years, 68% male, 32% female). Forty percent of all patients experienced the primary composite endpoint. Multivariable logistic regression found that only the presence of pancreatitis or abdominal infection at diagnosis was associated with an increased likelihood of experiencing thrombus progression in patients with untreated SVT (OR 3.61, P = 0.02). There was a statistically significant overall survival difference between patients that did and did not experience the primary composite endpoint after controlling for confounding variables. (p = 0.0068).
Conclusions:
Overall, only the presence of pancreatitis or intrabdominal infection were found to be significantly associated with thrombotic progression, with varices identified as marginally non-significant risk factor. Notably, thrombotic progression was associated with a significant reduction in overall survival.
Keywords: Anticoagulation, Cirrhosis, Portal vein thrombosis, Mortality
1. Introduction
Hepatic cirrhosis, the end stage of chronic liver disease, is a leading cause of morbidity and mortality in the United States [1]. The hematologic changes associated with cirrhosis result in a tenuously rebalanced hemostasis that carries a heightened risk of both bleeding and thrombosis [2-4]. Although bleeding has traditionally been the primary concern in this setting, the sum effect of the derangements, coupled with systemic processes of reduced venous flow volume and endothelial activation, is often largely prothrombotic [5-8]. Hypercoagulability in the context of cirrhosis carries an increased risk of splanchnic vein thrombosis (SVT); the portal vein is most commonly affected [9], and portal vein thrombosis (PVT) is estimated to occur in up to 25% of patients with end stage liver disease [10]. Known risk factors for SVT in cirrhosis include elevated MELD score, older age, esophageal varices, malignancy, pancreatitis, cytopenias (thrombocytopenia, anemia, and leukopenia), and pro-thrombotic mutations [11-14].
Management of this common complication presents a clinical dilemma. Although evidence and clinical judgement supports therapeutic anticoagulation in certain populations (i.e., patients listed for liver transplant and symptomatic patients), evidence guiding anticoagulant management for the broader population of patients with cirrhosis and SVT is not definitive. Namely, although PVT has not been shown to be clearly associated with mortality or liver disease progression in cirrhosis [15-20], most studies examining recanalization have identified potential morbidity and mortality benefits of treating PVT [16,21-23]. This suggests that in select patients, anticoagulation is beneficial and PVT is not fully benign. Studies have also consistently demonstrated that treatment of SVT with anticoagulation is broadly effective in achieving recanalization, and does not carry an increased risk of bleeding [24-26]. However, in the absence of data to aid in the identification of patients that are most likely to benefit, clinicians continue to have concerns regarding the risk of catastrophic hemorrhage. This is reflected by a lack of consensus in clinical guidelines with some expert panels recommending treatment only in symptomatic patients or patients listed for transplant, and others recommending a more global approach [27-31]. Given the paucity of high-level evidence, treatment decisions are largely based on clinician gestalt.
There is an unmet need for evidence-based guidance regarding the treatment of SVT in patients with cirrhosis who are asymptomatic and not listed for transplant. Multiple studies have attempted to provide guidance through identifying predictors of spontaneous recanalization and thereby determining patients who do not require treatment. However, aside from Maruyama et al. who identified specific parameters in the largest collateral vessel as negatively associated with spontaneous improvement of PVT in univariate analysis [32], no statistically significant predictors of recanalization have been identified to our knowledge [17,33,34]. This study aims to take an alternate approach by identifying predictors of thrombotic progression and other adverse thrombotic outcomes in patients with cirrhosis who were not treated with anticoagulation, thereby identifying patients who are most likely to benefit from therapeutic anticoagulation.
2. Methods
2.1. Study design and cohort selection
We conducted a single-center retrospective cohort study including adult patients with a history of cirrhosis and SVT diagnosis between January 2015 and December 2020 at Oregon Health and Science University. Only patients who did not receive initial treatment with anticoagulation at the time of SVT diagnosis were included. The study design was approved by the Institutional Review Board at OHSU prior to initiation (OHSU IRB number- STUDY00022298). Patients were identified using relevant diagnostic codes for SVT. Medical records of all consecutive patients from January 2015 to December 2020 were reviewed for inclusion. SVT was defined as any thrombus occurring in the splanchnic venous circulation including the portal vein, mesenteric veins, and splenic vein. SVT was confirmed by chart review, and the index day 0 of the study was the date of the first radiologic study showing evidence of SVT. Radiologic studies used to diagnose SVT included computed tomography and abdominal ultrasound, no distinction based on imaging modality was made. In some cases, upon further chart review, this occurred prior to January 2015, and these patients were not excluded. Following individual chart review, patients who did not have an imaging-confirmed diagnosis of SVT, were less than 18 years of age, had no history of cirrhosis, died within 30 days of SVT diagnosis, or were treated with anticoagulation at the time of original diagnosis were excluded.
2.2. Data collection and definitions
Included patients were assessed by individual chart review. Multiple variables were collected at the time of SVT diagnosis including pertinent demographic information (age, sex), etiology of cirrhosis (alcoholic, viral, etc), clinical indices (body mass index, use of antiplatelet agents), Model for End Stage Liver Disease (MELD) score, known varices, history of encephalopathy, history of ascites, thrombotic risk factors (smoking status, malignancy, pancreatitis, prior history of venous thrombosis or thromboembolism), and characterizations of the SVT (location, tumor thrombus, symptomatic, obstructive). Pertinent laboratory values at the time of SVT diagnosis were collected including sodium, creatinine, glomerular filtration rate, total bilirubin, hemoglobin, platelet, international normalized ratio, partial thromboplastin time, and fibrinogen. After the time of original diagnosis, imaging reports were reviewed to assess for imaging-confirmed SVT progression, recanalization, and cavernous transformation. Imaging records and chart notes were also assessed for new venous thrombosis or thromboembolism (VTE) including deep vein thrombosis (DVT) and pulmonary embolism (PE) occurring after the original PVT diagnosis. Data on portal cholangiopathy occurring after diagnosis was collected. Charts were also reviewed for subsequent arterial events including myocardial infarction (MI), cerebrovascular vascular accident (CVA), and intestinal ischemia or infarction. Charts with missing data were excluded from the analysis.
2.3. Outcome definitions
The primary composite endpoint was defined as one or more of the following: imaging-confirmed SVT progression, development of cavernous transformation, intestinal ischemia, portal cholangiopathy, or new venous or arterial thrombosis. This composite endpoint was chosen to include the most common and most devastating potential thrombotic complications that would reasonably have been prevented with anticoagulation. A composite outcome was favored to avoid under-identification of potential anticoagulant benefit given that each component was considered an adverse outcome warranting prevention. To allow for accurate assessment of global thrombotic progression, including thromboses outside of the splanchnic venous system, thrombotic outcomes occurring the setting of malignant splanchnic vascular invasion were not excluded. SVT progression was defined as new thrombosis extending from the original clot, enlargement of original clot, or progression from non-occlusive to occlusive thrombi, as documented in radiologic reports. Recanalization was defined as either imaging reported recanalization or new patency of the previously thrombosed vessel as documented in radiologic reports. Cavernous transformation was defined as new imaging-confirmed documentation of cavernous transformation occurring on imaging done after the initial SVT diagnosis as documented in radiologic reports. New VTE was defined as new SVT, DVT or PE. DVT and PE were defined by identification of such within a radiographic report, or if described as a new diagnosis in subsequent chart review. Imaging reports were followed through December 2021, or one year following the end of the study's enrolment period (December 2020). All patient deaths occurring in this time frame were also recorded.
2.4. Statistical methods
Baseline demographic characteristics were analyzed using descriptive statistics. Descriptive analysis, univariate logistic regression, and multivariable logistic regression were performed in STATA version 12.1 and R (R core team 2019). Logistic regression was performed in python 3.6 using the scikit-learn Logistic Regression module. Predictive performance of the logistic regression model was evaluated using 20-fold cross validation. Because it was not possible to accurately associate deaths that occurred in the acute setting of SVT diagnosis with the composite outcome of interest, only deaths occurring after 30 days of initial diagnosis were included to allow reasonable association with thrombotic progression.
3. Results
3.1. Cohort characteristics
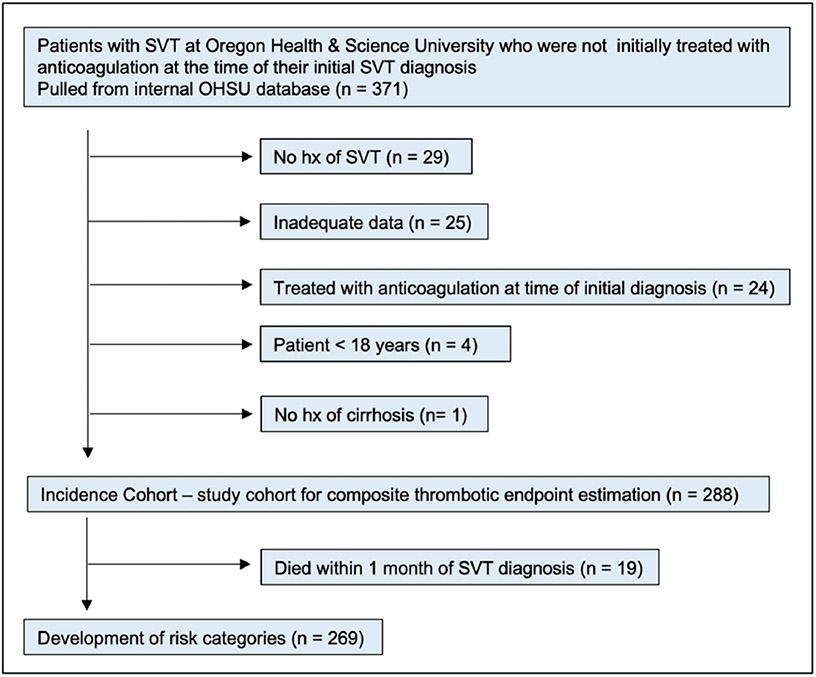
A total of 371 patients were initially identified, of which 83 patients were found not to meet study inclusion criteria (Fig. 1). Of 288 remaining, 19 patients were excluded due to death within 30 days of SVT diagnosis. A total of 269 patients were included and assessed for the primary composite clinical endpoint.
Fig. 1.
Study flow diagram with inclusion and exclusion criteria for the cumulative incidence and RAM cohort.
Study patient demographics, along with the underlying etiology of liver disease, clinical indices of risk, location of thrombus, and select laboratory values are summarized in Table 1. Of the 269 included patients, 183 were male, while 86 patients were female. The most common etiologies of underlying liver disease reflected in the cohort were multifactorial (28.9%), viral hepatitis (27.1%), alcoholic cirrhosis (17.1%), and non-alcoholic steatohepatitis (NASH) (13.4%), other/cryptogenic (8.6%), and autoimmune (4.8%). Of the clinical indices recorded, patients with varices or ascites at the time of diagnosis were highly represented (61.7% and 57.6%, respectively). A large proportion of patients had thrombi of the portal vein alone (78.1%). Finally, values for components of the MELD-Na score were not dissimilar between patients with and without the composite endpoint (average MELD score with composite endpoint 14; average MELD score without composite endpoint 14).
Table 1.
Clinical and demographic information for patients with splanchnic vein thrombosis.
| Total | No composite event |
Composite event |
|
|---|---|---|---|
| Patients | 269 | 162 | 107 (40%) |
| Sex, n (%) | |||
| Male | 183 (68%) | 109 | 74 (40%) |
| Female | 86 (32%) | 53 | 33 (38%) |
| Age, median (IQR) | 61 (54–66) | 60 (54–65) | 62 (56–66) |
| Etiology of liver disease | |||
| Viral hepatitis | 73 (27%) | 48 | 25 (34%) |
| Alcoholic cirrhosis | 46 (17%) | 28 | 18 (39%) |
| NASH | 36 (13%) | 19 | 17 (47%) |
| Autoimmune | 13 (5%) | 9 | 4 (31%) |
| Multifactorial | 78 (29%) | 42 | 36 (46%) |
| Other or unknown | 23 (9%) | 16 | 7 (30%) |
| Clinical indices | |||
| Smoker | 66 (25%) | 39 | 27 (41%) |
| History of VTEa | 18 (7%) | 9 | 10 (56%) |
| Varicesa | 166 (62%) | 93 | 73 (44%) |
| Ascitesa | 155 (58%) | 92 | 63 (41%) |
| Encephalopathya | 63 (24%) | 38 | 25 (40%) |
| Tumor thrombus | 81 (30%) | 48 | 33 (41%) |
| Obstructive clota | 84 (32%) | 45 | 39 (46%) |
| Aspirin use | 26 (10%) | 14 | 12 (46%) |
| MELD, median (IQR) | 14 (10–19) | 14 (10–19) | 14 (11–19) |
| BMI, mean (SD) | 29.5 (6.7) | 29.9 (6.7) | 28.9 (6.8) |
| Location of thrombus | |||
| Portal vein | 210 (78%) | 130 | 80 (38%) |
| Superior mesenteric vein | 5 (2%) | 4 | 1 (20%) |
| Multiple splanchnic | 47 (17%) | 25 | 22 (47%) |
| veins | |||
| Other | 7 (3%) | 3 | 4 (57%) |
| Lab values, mean (SD) | |||
| Na | 136 (5) | 136 (5) | 137 (4) |
| Cr | 1.0 (0.7) | 1.1 (0.8) | 1.0 (0.6) |
| INR | 1.4 (0.7) | 1.5 (0.8) | 1.4 (0.3) |
| Platelet count | 112 (87) | 109 (65) | 117 (113) |
| Total bilirubin | 3.2 (5.8) | 3.8 (6.9) | 2.2 (3.1) |
| Hgb | 11.8 (2.5) | 11.8 (2.6) | 11.8 (2.4) |
As documented at time of diagnosis.
3.2. Summary of the composite thrombotic endpoint
The proportion of each clinical outcome encompassing the primary composite endpoint are reported in Table 2. Components of the composite outcome were not mutually exclusive and the most common outcomes of patients with reported positive composite endpoint were that of clot enlargement (52%), development of cavernous thrombosis (28%), and progression of the original thrombus from non-occlusive to occlusive (23%). Forty percent of all patients experienced the primary composite endpoint (40% of men and 38% of women in the cohort).
Table 2.
Frequency of composite events.
| Event type | Count | % of events | % of cohort |
|---|---|---|---|
| Clot enlargement | 56 | 52% | 21% |
| Cavernous thrombosis | 30 | 28% | 11% |
| Progression to occlusion | 25 | 23% | 9% |
| Additional venous thrombosis | 19 | 18% | 7% |
| Arterial thrombosis | 13 | 12% | 5% |
| Intestinal ischemia | 12 | 11% | 4% |
| Portal cholangiopathy | 12 | 11% | 4% |
| Total | 167a | – | – |
Some patients developed multiple events of interest; 167 events occurred across 107 patients. Percentage of total given as percent of individuals developing an outcome of interest (n = 107), percentages will not sum to 100.
3.3. Covariate analysis reveals pancreatitis increased the probability of the primary composite endpoint
Using multivariable logistic regression, odds ratios (OR) were calculated from relevant demographic data, liver disease etiology, thrombus location and laboratory values relative to the primary composite endpoint (Table 3). Variables that met statistical significance at the time of SVT diagnosis included concurrent pancreatitis or intrabdominal infection (OR 3.61, CI 1.21–10.71, p = 0.02). The remainder of comparisons across covariates, including presence of varices at diagnoses (p = 0.07) and underlying etiology of cirrhosis (multifactorial p = 0.09, and autoimmune etiologies p = 0.07), did not meet statistical significance.
Table 3.
Multivariable logistic regression model of predictors of composite thrombotic endpoint.
| Covariates | Adj OR |
Unadj OR |
95% CI |
p | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age | 1.00 | 0.99 | 0.97 | 1.03 | 0.92 |
| Gender (female) | 1.12 | 0.99 | 0.59 | 2.08 | 0.73 |
| BMI | 0.98 | 0.98 | 0.94 | 1.03 | 0.47 |
| History of VTE | 1.49 | 1.62 | 0.45 | 4.98 | 0.51 |
| Aspirin use | 1.78 | 1.38 | 0.68 | 4.65 | 0.24 |
| Etiology | |||||
| Viral | 0.61 | 0.88 | 0.21 | 1.76 | 0.36 |
| Alcoholic | 0.36 | 0.69 | 0.08 | 1.65 | 0.19 |
| Multifactorial | 0.43 | 0.75 | 0.16 | 1.14 | 0.09 |
| Autoimmune | 0.30 | 0.57 | 0.08 | 1.10 | 0.07 |
| Other/unknown | 0.75 | 1.56 | 0.29 | 1.89 | 0.54 |
| Varices | 1.81 | 1.72 | 0.94 | 3.47 | 0.07 |
| Tumor-associated thrombus | 1.30 | 1.15 | 0.60 | 2.79 | 0.50 |
| Malignancy | 0.87 | 0.91 | 0.43 | 1.76 | 0.69 |
| Pancreatitis or intra-abdominal infection | 3.61 | 3.23 | 1.21 | 10.71 | 0.02 |
| Location | |||||
| Portal vein | 0.38 | 0.39 | 0.04 | 3.81 | 0.41 |
| Multiple splanchnic veins | 1.11 | 1.49 | 0.53 | 2.35 | 0.77 |
| Other | 2.48 | 2.42 | 0.37 | 16.56 | 0.35 |
| Obstructive clot | 1.49 | 1.36 | 0.78 | 2.86 | 0.23 |
| Ascites | 0.98 | 1.22 | 0.52 | 1.87 | 0.96 |
| Na | 0.99 | 0.99 | 0.98 | 1.02 | 0.98 |
| Cr | 0.79 | 0.67 | 0.48 | 1.32 | 0.38 |
| Total bilirubin | 0.90 | 0.90 | 0.82 | 1.00 | 0.5 |
3.4. The primary composite endpoint was significantly associated with worsened overall survival
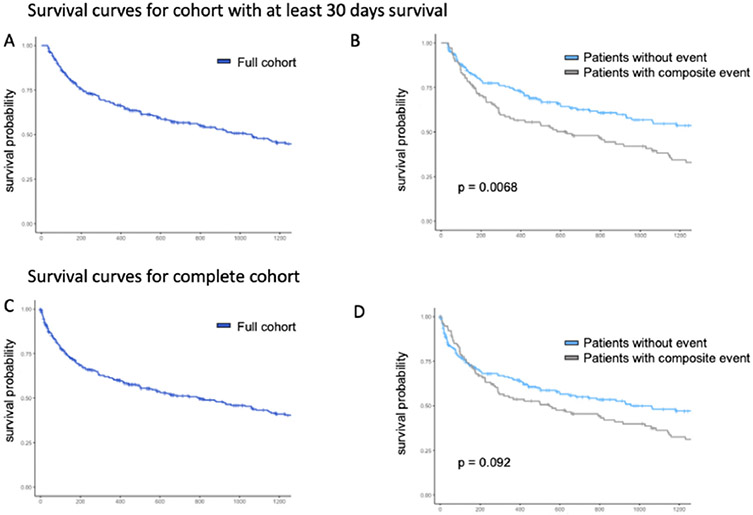
Overall survival (OS) was calculated across the defined study cohort as a whole and demonstrated <25% survival across all study patients after approximately 8 years (3000 days) (Fig. 2). The median overall survival was 2.7 years. When OS was compared between patients with and without clinical outcomes encompassing the primary composite outcome, there was a statistically significant difference between groups (p = 0.0068). Overall survival was also examined in the cohort without mortality restriction in the acute post-diagnostic period. For this cohort, median survival time was 2.2 years. In this cohort, the clinical outcomes did not predict a significant difference in survival (p = 0.092).
Fig. 2. Kaplan-Meier Survival Curves for Patients with Splanchnic Vein Thrombosis.
Kaplan-Meier survival curves are provided for the cohort of patients who survived a minimum of 30 days with splanchnic vein thrombosis (A) and grouped by composite outcome (B). Survival curves are also demonstrated for the cohort inclusive of mortality <30 days with splanchnic vein thrombosis (C) and grouped by composite outcome (D).
4. Discussion
In this study of patients with cirrhosis and SVT, 40% of patients developed thrombotic progression. Only the presence of pancreatitis or intrabdominal infection was significantly associated with increased risk of thrombotic outcomes (p = 0.02). We observed a significant predictive association between the primary endpoint and reduced overall survival (p = 0.0068).
Previous reported rates of PVT progression in patients with cirrhosis range from 7 to 48% [17,18,21,32,35,36]. It was not unexpected that our estimate for a composite outcome, which encompassed more than PVT progression alone, is on the higher end of this spectrum. Although thrombotic progression is known to be common, to our knowledge no studies have identified specific risk factors for VTE recurrence or PVT progression in patients with cirrhosis. In our evaluation of 21 objective clinical predictors, many known to be independently associated with increased thrombotic risk, we detected only one variable – the presence of pancreatitis or intrabdominal infection – that was significantly associated with increased risk of thrombotic outcomes. This may be due to inflammation driving clot progression. Splanchnic thrombosis is known to occur in up to 11–22% of cases of acute pancreatitis [37,38], with evidence that less than half of cases independently recanalize [38,39]. Some studies evaluating the utility of anticoagulation for SVT in the setting of pancreatitis have not shown significant differences in rates of PVT recanalization [38,39], while others have demonstrated that treatment significantly increase rates of recanalization (albeit with concomitant increases in GI bleeding risk) [40,41].
Although not significant in this analysis, in multiple retrospective and prospective studies, varices have been identified as the strongest or only predictor of PVT development and progression [15,32,42,43]. Varices can be viewed as a marker of hemodynamic dysregulation and reduced splanchnic vein flow velocity. The clear significance of varices in the literature, coupled with a lack of significance from markers of hepatic function in this and other studies suggests that in patients with cirrhosis, hemodynamic risks may outweigh those posed by synthetic liver dysfunction. Clinically, the link between varices and SVT development and/or progression is significant in that patients with cirrhosis and PVT who are treated with anticoagulation have decreased rates of variceal bleeding [35,44]. It is theorized that PVT may contribute to worsening portal hypertension and variceal rupture. Classically, varices have been viewed as a reason to withhold anticoagulation, however the increasingly recognized importance of varices as a contributor to thrombotic pathogenesis suggests varices should perhaps be considered as a reason to initiate anticoagulation.
To our knowledge, this is the first study evaluating predictors associated with thrombus progression. Prior studies attempting the converse of what is being done here, namely to identify predictors of spontaneous recanalization, have also largely failed to identify significant variables. Demographic variables including age and sex, and clinical variables including severity of renal and hepatic dysfunction, ascites, location of thrombus, and cavernous transformation have not been found to be significantly predictive of recanalization [45]. In either case, the results of this analysis do align with current practice in that case-by-case clinician gestalt remains most useful guide for initiation of anticoagulation. Ultimately, there remains a need for randomized, control trials to adequately assess risk of progression in this tenuous patient population.
Another important finding in this study was a significant predictive association between the primary composite endpoint and diminished overall survival. This relationship was not present when analysis was subsequently performed in a group without mortality restrictions for inclusion, suggesting that there is a subset of patients who die in the acute post-diagnostic without adequate follow-up time to determine their true risk of developing the clinical complications of interest to this investigation. Available data examining the mortality implications of splanchnic thrombus progression in patients with cirrhosis is overall limited. One prospective study of 22 patients with cirrhosis and untreated non-malignant PVT found that progression of PVT on follow-up imaging was associated with significantly higher rates of mortality and hepatic decompensation [21]. In contrast, a retrospective study of 42 patients with untreated non-malignant PVT showed no survival impact of PVT progression [17,46]. Beyond these studies, to our knowledge minimal data exists regarding the mortality implications of SVT or other thrombotic progression in patients with cirrhosis not undergoing liver transplant. More broadly, literature examining the mortality implications of SVT in cirrhosis are also contradictory. While multiple studies have found that the presence vs. absence of PVT in cirrhosis does not affect mortality [17-20,47,48], others have demonstrated that PVT treatment and recanalization carries a mortality benefit [16,21-23,49]. Ultimately, the known benefit of recanalization along with the findings presented here may suggest that treatment of splanchnic thrombosis with anticoagulation provides a mortality benefit for at least a subset of patients. While this finding is notable, an important possibility is that patients with more advanced cirrhosis may be at higher risk for thrombus progression, and as such the progression of thrombosis itself may not be the causal factor driving diminished survival.
There are limitations in this study. The retrospective design without prospective enrolment or random assignment of medical intervention carries inherent bias in terms of internal validity. This is especially true in the case of SVT, which may persist asymptomatically for a long period of time before manifesting symptoms or being incidentally discovered. In this setting it is difficult to clearly identify the true time at which many thromboses in this study developed. Retrospective data collection also limits data availability and accuracy in that patients included in this study did not have uniform follow up imaging, meaning that in many cases repeat imaging was prompted by decompensation, symptoms, or assessment of major comorbidities including malignancy. As such, in case of both initial and follow up imaging, the retrospective design creates a potentially skewed sample in which patients more likely to experience negative outcomes may have been preferentially included and followed. Another potentially confounding element is the selective inclusion only of patients who were not treated with anticoagulation. It may be assumed that patients not treated with anticoagulation harbored at least some objective or clinical/subjective contraindications to anticoagulation, perhaps biasing this sample toward patients either already prone to bleeding or otherwise more decompensated. That this patient population was not compared to those who were treated with anticoagulation, limits the broad generalizability of the findings to all patients with cirrhosis and SVT. Additionally, the single-center nature of this study limits external validity. Strengths of this study include uniform, randomized patient selection, accurate methodology with all data verified by individual chart review, and novel assessment of heretofore not previously examined thrombotic outcomes in patients with cirrhosis.
As discussed above, future research to successfully identify patients with cirrhosis and SVT who will benefit from anticoagulation is needed. Prospective observational studies with pre-determined imaging and follow up schedules, and therefore more uniform and complete data sets, are needed to provide the most accurate information regarding the natural history of this common condition and the best basis for management guidance. More complete data may be achieved via multi-institution research collaboratives for SVT aimed toward the design and completion of randomized controlled trials. More comprehensive data may even allow for the derivation and validation of a predictive model to aid clinicians in identifying patients who would benefit from anticoagulation. Evaluation of thrombotic risk and strategies for mitigation thereof are also notable avenues of future research.
In conclusion, this study identified pancreatitis or intrabdominal infection as predictive of worsening thrombotic outcomes in patients with cirrhosis and SVT. This study also showed that the development of progressive thrombotic events in patients with untreated SVT and cirrhosis portends a poorer overall survival. This speaks to potential benefits of therapeutic anticoagulation in this patient population and the need for accurate predictive models to identify patients most likely to benefit.
Grant support
J. Shatzel is supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (HL151367).
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Tapper EB, Parikh ND, Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study, BMJ 362 (2018), k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McMurry H, Jou J, Shatzel J, The hemostatic and thrombotic complications of liver disease, Eur. J. Haematol 107 (4) (2021) 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kujovich JL, Coagulopathy in liver disease: a balancing act, Hematology 2015 (2015) 243–249. [DOI] [PubMed] [Google Scholar]
- [4].Tripodi A, Mannucci PM, The coagulopathy of chronic liver disease, N. Engl. J. Med 365 (2011) 147–156. [DOI] [PubMed] [Google Scholar]
- [5].Tripodi A, Anstee QM, Sogaard KK, Primignani M, Valla DC, Hypercoagulability in cirrhosis: causes and consequences, J. Thromb. Haemost 9 (2011)1713–1723. [DOI] [PubMed] [Google Scholar]
- [6].Ng KJ, Lee YK, Huang MY, Hsu CY, Su YC, Risks of venous thromboembolism in patients with liver cirrhosis: a nationwide cohort study in Taiwan, J. Thromb. Haemost 13 (2015) 206–213. [DOI] [PubMed] [Google Scholar]
- [7].Sogaard KK, Horvath-Puho E, Gronbaek H, Jepsen P, Vilstrup H, Sorensen HT, Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study, Am. J. Gastroenterol 104 (2009) 96–101. [DOI] [PubMed] [Google Scholar]
- [8].Mantaka A, Augoustaki A, Kouroumalis EA, Samonakis DN, Portal vein thrombosis in cirrhosis: diagnosis, natural history, and therapeutic challenges, Ann. Gastroenterol 31 (2018) 315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Valeriani E, Riva N, Di Nisio M, Ageno W, Splanchnic vein thrombosis: current perspectives, Vasc. Health Risk Manag 15 (2019) 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fimognari FL, Violi F, Portal vein thrombosis in liver cirrhosis, Intern. Emerg. Med 3 (2008) 213–218. [DOI] [PubMed] [Google Scholar]
- [11].Sogaard KK, Astrup LB, Vilstrup H, Gronbaek H, Portal vein thrombosis; risk factors, clinical presentation and treatment, BMC Gastroenterol. 7 (2007), 34–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Turon F, Driever EG, Baiges A, et al. , Predicting portal thrombosis in cirrhosis: a prospective study of clinical, ultrasonographic and hemostatic factors, J. Hepatol 75 (2021) 1367–1376. [DOI] [PubMed] [Google Scholar]
- [13].Amitrano L, Anna Guardascione M, Brancaccio V, et al. , Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis, J. Hepatol 40 (2004) 736–741. [DOI] [PubMed] [Google Scholar]
- [14].Nery F, Carneiro P, Correia S, et al. , Systemic inflammation as a risk factor for portal vein thrombosis in cirrhosis: a prospective longitudinal study, Eur. J. Gastroenterol. Hepatol 33 (2021) e108–e113. [DOI] [PubMed] [Google Scholar]
- [15].Nery F, Chevret S, Condat B, et al. , Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study, Hepatology 61 (2015) 660–667. [DOI] [PubMed] [Google Scholar]
- [16].Noronha Ferreira C Reis D Cortez-Pinto H et al. Anticoagulation in Cirrhosis and Portal Vein Thrombosis Is Safe and Improves Prognosis in Advanced Cirrhosis. Digestive Diseases and Sciences;64:2671–2683. [DOI] [PubMed] [Google Scholar]
- [17].Luca A, Caruso S, Milazzo M, et al. , Natural course of extrahepatic nonmalignant partial portal vein thrombosis in patients with cirrhosis, Radiology 265 (2012) 124–132. [DOI] [PubMed] [Google Scholar]
- [18].Chen Z, Ran T, Cao H, Xu F, Zhou Z.-h., He S, The impact of portal vein thrombosis on the prognosis of patients with cirrhosis: a retrospective propensity-score matched study, Front. Med 8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xian J, Tang Y, Shao H, Wang X, Zhang M, Xing T, Effect of portal vein thrombosis on the prognosis of patients with cirrhosis without a liver transplant: a systematic review and meta-analysis, Medicine 100 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Berry K, Taylor J, Liou IW, Ioannou GN, Portal vein thrombosis is not associated with increased mortality among patients with cirrhosis, Clin. Gastroenterol.Hepatol 13 (2015) 585–593. [DOI] [PubMed] [Google Scholar]
- [21].Girleanu I, Stanciu C, Cojocariu C, Boiculese L, Singeap A-M, Trifan A, Natural course of nonmalignant partial portal vein thrombosis in cirrhotic patients, Saudi J. Gastroenterol 20 (2014) 288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pettinari I, Vukotic R, Stefanescu H, et al. , Clinical impact and safety of anticoagulants for portal vein thrombosis in cirrhosis, Off. J. Am. Coll. Gastroenterol 114 (2019). [DOI] [PubMed] [Google Scholar]
- [23].Senzolo M, Riva N, Dentali F, et al. , Long-term outcome of splanchnic vein thrombosis in cirrhosis, Clin. Transl. Gastroenterol 9 (2018) 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Qi X, De Stefano V, Li H, Dai J, Guo X, Fan D, Anticoagulation for the treatment of portal vein thrombosis in liver cirrhosis: a systematic review and meta-analysis of observational studies, Eur.J.Intern.Med 26 (2015) 23–29. [DOI] [PubMed] [Google Scholar]
- [25].Wang L, Guo X, Xu X, et al. , Anticoagulation favors thrombus recanalization and survival in patients with liver cirrhosis and portal vein thrombosis: results of a meta-analysis, Adv. Ther 38 (2021) 495–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ghazaleh S, Beran A, Aburayyan K, et al. , Efficacy and safety of anticoagulation in non-malignant portal vein thrombosis in patients with liver cirrhosis: a systematic review and meta-analysis, Ann. Gastroenterol 34 (2021) 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Simonetto DA, Singal AK, Garcia-Tsao G, Caldwell SH, Ahn J, Kamath PS, ACG clinical guideline: disorders of the hepatic and mesenteric circulation, Am. J. Gastroenterol 115 (2020) 18–40. [DOI] [PubMed] [Google Scholar]
- [28].de Franchis R, Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension, J. Hepatol 63 (2015) 743–752. [DOI] [PubMed] [Google Scholar]
- [29].European Association for the Study of the Liver, Electronic address eee. EASL clinical practice guidelines: vascular diseases of the liver, J. Hepatol 64 (2016) 179–202. [DOI] [PubMed] [Google Scholar]
- [30].Yoshiji H, Nagoshi S, Akahane T, et al. , Evidence-based clinical practice guidelines for liver cirrhosis 2020, J. Gastroenterol 56 (2021) 593–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Northup PG, Garcia-Pagan JC, Garcia-Tsao G, et al. , Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 practice guidance by the American Association for the Study of Liver Diseases, Hepatology 73 (2021) 366–413. [DOI] [PubMed] [Google Scholar]
- [32].Maruyama H, Okugawa H, Takahashi M, Yokosuka O, De novo portal vein thrombosis in virus-related cirrhosis: predictive factors and long-term outcomes, Am. J. Gastroenterol 108 (2013) 568–574. [DOI] [PubMed] [Google Scholar]
- [33].Qi X, Guo X, Yoshida EM, et al. , Transient portal vein thrombosis in liver cirrhosis, BMC Med. 16 (2018) 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chen H, Liu L, Qi X, et al. , Efficacy and safety of anticoagulation in more advanced portal vein thrombosis in patients with liver cirrhosis, Eur. J. Gastroenterol. Hepatol 28 (2016) 82–89. [DOI] [PubMed] [Google Scholar]
- [35].Loffredo L, Pastori D, Farcomeni A, Violi F, Effects of anticoagulants in patients with cirrhosis and portal vein thrombosis: a systematic review and meta-analysis, Gastroenterology 153 (480–487) (2017), e481. [DOI] [PubMed] [Google Scholar]
- [36].Acuna-Villaorduna A, Tran V, Gonzalez-Lugo JD, Azimi-Nekoo E, Billett HH, Natural history and clinical outcomes in patients with portal vein thrombosis by etiology: a retrospective cohort study, Thromb. Res 174 (2019) 137–140. [DOI] [PubMed] [Google Scholar]
- [37].Xu W, Qi X, Chen J, Su C, Guo X, Prevalence of splanchnic vein thrombosis in pancreatitis: a systematic review and meta-analysis of observational studies, Gastroenterol. Res. Pract 2015 (2015), 245460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Junare PR, Udgirkar S, Nair S, et al. , Splanchnic venous thrombosis in acute pancreatitis: does anticoagulation affect outcome? Gastroenterol.Res. 13 (2020) 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gonzelez HJ, Sahay SJ, Samadi B, Davidson BR, Rahman SH, Splanchnic vein thrombosis in severe acute pancreatitis: a 2-year, single-institution experience, HPB (Oxford) 13 (2011) 860–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Anderson W, Niccum B, Chitnavis M, Uppal D, Hays AR, Outcomes of anticoagulation for portal and/or splenic vein thrombosis in setting of acute pancreatitis: 11, Off. J. Am. Coll. Gastroenterol 112 (2017). [Google Scholar]
- [41].Sissingh NJ, Groen JV, Koole D, et al. , Therapeutic anticoagulation for splanchnic vein thrombosis in acute pancreatitis: a systematic review and meta-analysis, Pancreatology 22 (2) (2021) 235–243. [DOI] [PubMed] [Google Scholar]
- [42].Stine JG, Wang J, Shah PM, et al. , Decreased portal vein velocity is predictive of the development of portal vein thrombosis: a matched case-control study, Liver Int. 38 (2018) 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zocco MA, Di Stasio E, De Cristofaro R, et al. , Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development, J. Hepatol 51 (2009) 682–689. [DOI] [PubMed] [Google Scholar]
- [44].Senzolo M, T MS, Rossetto V, et al. , Prospective evaluation of anticoagulation and transjugular intrahepatie portosystemic shunt for the management of portal vein thrombosis in cirrhosis, Liver Int. 32 (2012) 919–927. [DOI] [PubMed] [Google Scholar]
- [45].Faccia M, Ainora ME, Ponziani FR, et al. , Portal vein thrombosis in cirrhosis: why a well-known complication is still matter of debate, World J. Gastroenterol 25 (2019) 4437–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].. !!! INVALID CITATION !!! 13.
- [47].Nery F, Chevret S, Condat B, et al. , Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study, Hepatology 61 (2015) 660–667. [DOI] [PubMed] [Google Scholar]
- [48].. !!! INVALID CITATION !!! 11-16.
- [49].Valeriani E, Di Nisio M, Riva N, et al. , Anticoagulant therapy for splanchnic vein thrombosis: a systematic review and meta-analysis, Blood 137 (2021) 1233–1240. [DOI] [PubMed] [Google Scholar]