Extended Data Figure 3: Surface properties of GATOR2.
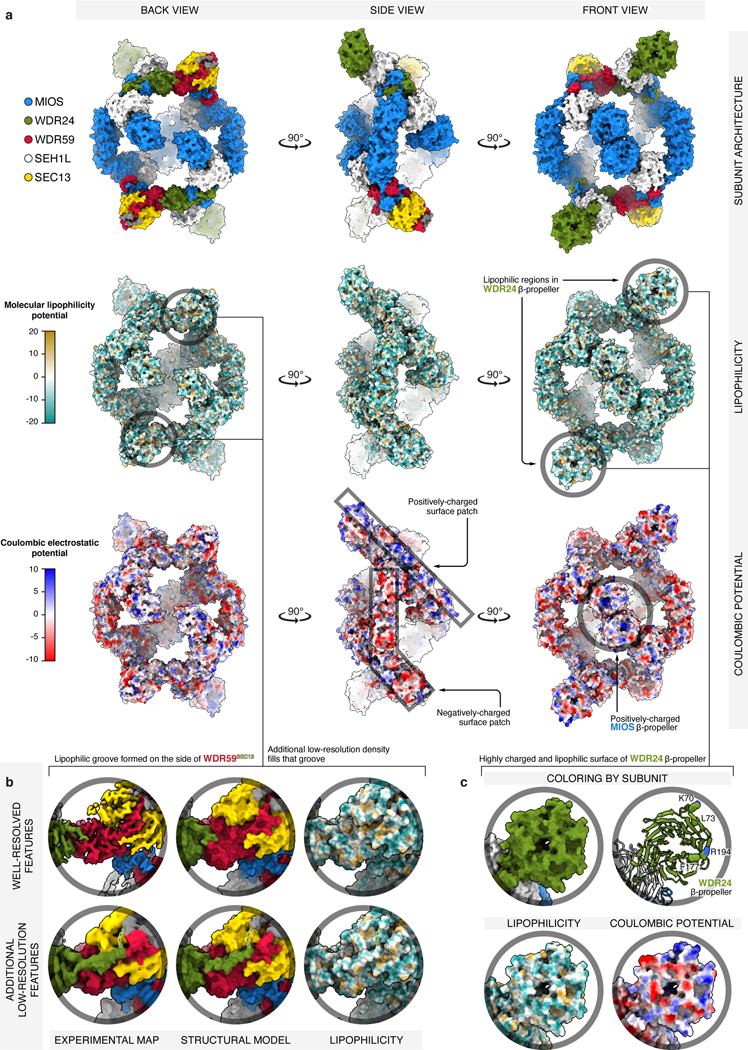
a) Three orthogonal views of GATOR2 are shown in surface representation and colored by: subunit identity (top), molecular lipophilicity potential (middle), coulombic electrostatic potential (bottom). Surface calculations were performed in ChimeraX55, with atomic lipophilicity values from Ghose et al.56 The most lipophilic surfaces are drawn as orange and the least lipophilic as cyan. Negatively charged surfaces are drawn as red, and positively as blue. GATOR2 is highly charged at its surface, with the only exposed lipophilic patches of significant size found near the donor β-blade of WDR59, and the β-propeller of WDR24 (see panels (b) and (c) for details). Furthermore, there are two major surface patches where either negative or positive charge dominates. The mostly-positive patch stretches from the side of the β-propeller of WDR24, through the WDR24-MIOS CTD, to MIOS glove β-propeller. The mostly-negative patch starts with the MIOS α-solenoid, passes through the MIOS-WDR59 CTD, and ends at the tip of SEC13. The center of MIOS β-propellers appears highly positively charged.
(b) Detailed view of the lipophilic patch near the donor β-blade of WDR59. While it might appear that the surface of the WDR59 β-blade forms a lipophilic groove, that groove is in fact occupied by protein density extending from the α-solenoid of WDR24. This WDR24 density likely continues to the top of the SEC13WDR59 β-propeller (green dashed line), where we found two better-resolved stretches of amino-acid sequence (colored in gray). Yet, for these particular sections of GATOR2, we were unable to build a high-confidence structural model or to unambiguously deduce the amino-acid sequence.
(c) Detailed view of the highly lipophilic and charged surface of the WDR24 β-propeller. The WDR24 β-propeller contains strong patches of negatively and positively charged surface. There are additional sporadic sections of highly lipophilic surface. Residues predicted by the DREAMM algorithm as membrane-penetrating are drawn as spheres57.
