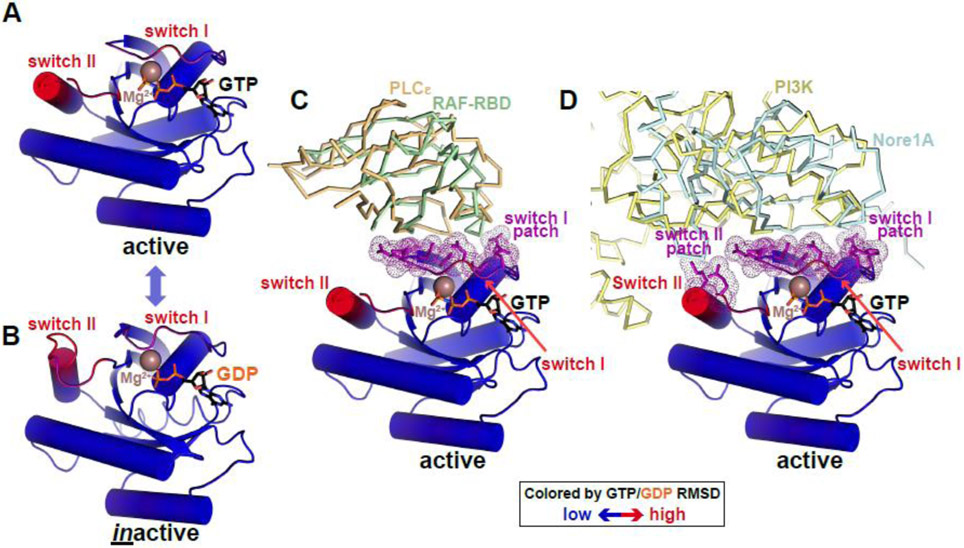
Figure 3. Conformational switching in RAS: an analogy for pseudokinases?
(A,B) RAS as a prototypical G protein conformational switch. RAS is represented in cartoon form, colored according to backbone position RMSD when GTP- and GDP-bound forms are overlaid (blue is no difference, red is maximum difference: ~5Å). Regions undergoing the greatest conformational changes were originally named switch I and switch II [107] – as labelled. Bound nucleotides are shown in black sticks and marked, and Mg2+ ions are brown spheres. Active RAS is from PDB ID 4G0N [108] and inactive RAS from PDB ID 4Q21 [107].
(C) Active GTP-bound RAS binds effector proteins phospholipase Cε and RAF (through its RAS-binding domain or RBD) via switch I. Interfacial residues in RAS are colored purple, and correspond with switch I. PLCε is shown as orange ribbons (PBD ID: 2C5L [109]) and RAF-RBD as green ribbons (PDB ID: 4G0N [108]).
(D) Active GTP-bound RAS uses both switch I and switch II to bind phosphatidylinositol-3-kinase (PI3K) and Nore1A, with interfacial residues (purple) coinciding with the two switch regions just as the kinase αC patch coincides with regions of maximum change in the kinase N-lobe. PI3K is shown in pale yellow ribbons (PDB ID: 1HE8 [110]) and Nore1A is shown as pale blue ribbons (PDB ID: 3DDC [111]).