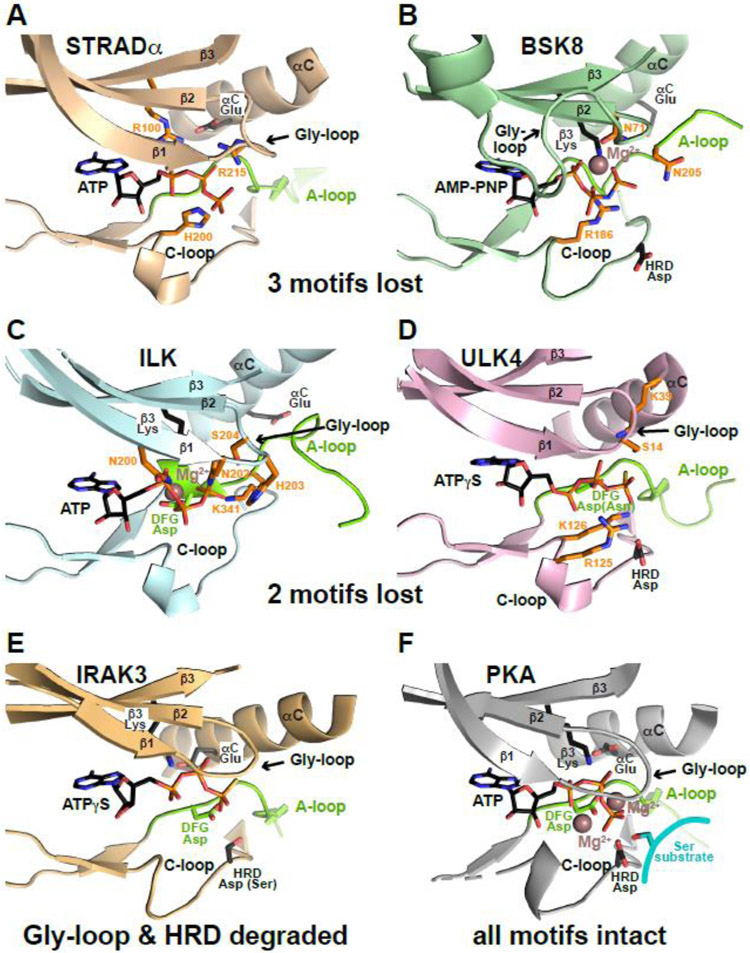
Figure 4. Resilience of pseudokinase ATP binding.
ATP binding sites are shown for a series of pseudokinase domains that retain ATP binding (A-E) and for PKA (F) for comparison. Bound ATP, AMP-PNP or ATPγS is shown. The part of the activation loop shown is colored green (as is the DFG Asp when present), and preserved ATP-interacting side-chains are shown in black sticks. ‘Compensatory’ side-chains that make up for lost ATP-interacting residues by contacting the nucleotide as described in the text are colored orange. Mg2+ is shown as brown sphere in (B, C, F), but is absent in (A, D, E), which show cation-independent ATP binding.