Abstract
Background:
Female sex confers a survival advantage following severe injury in the setting of trauma-induced coagulopathy, with female platelets having a heightened responsiveness likely due to estrogen. The effects of testosterone on platelet biology are unknown, and platelets express both estradiol and androgen receptors on the plasma membrane. We hypothesize testosterone decreases platelet responses in vitro, and there are baseline differences in platelet function and metabolism stratified by sex/age.
Study Design and Methods:
Apheresis platelets were collected from: older males (OM) ≥45 years, younger males (YM) <45 years, older females (OF) ≥54 years, and younger females (YF) <54 years and testosterone and estradiol were measured. Platelets were incubated with testosterone [5.31 ng/ml], estradiol [105 pg/ml] or vehicle and stimulated with buffer, adenosine diphosphate [20 μM], platelet activating factor [2 μM], or thrombin [0.3 U/ml]. Aggregation, CD62P surface expression, fibrinogen receptor surface expression, and platelet mitochondrial metabolism were measured.
Results:
Testosterone significantly inhibited aggregation in OF and OM (p<0.05), inhibited CD41a expression in YF, YM, and OM (p<0.05), affected a few of the baseline amounts of CD62P surface expression but not platelet activation to PAF and ADP, and variably changed platelet metabolism.
Discussion:
Platelets have sex- and age-specific aggregation, receptor expression, and metabolism. Testosterone decreases platelet function dependent on the stimulus, age, and sex. Similarly, platelet metabolism has varying responses to sex hormones with baseline metabolic differences dependent upon sex and age.
Keywords: Sex dimorphisms, estradiol, testosterone, platelets, mitochondria
Introduction
All cellular members of the innate and adaptive immune systems have sex hormone receptors on the cell surface, and there are sex differences in immune responses to various diseases.1–3 Following injury, the female sex confers a survival advantage in the setting of trauma-induced coagulopathy, with female platelets having a heightened responsiveness to stimuli versus platelets from males.4 Female platelet responsiveness is thought to be mediated by estradiol, and studies examining the effect of testosterone on platelet biology are lacking.5 Platelets are part of the innate system and express both estradiol and androgen receptors on the plasma membrane.6 Furthermore, studies on the function and metabolism of human apheresis platelets are limited, especially looking at cohorts stratified by age and sex.
The purpose of this study is to further evaluate sex dimorphisms in apheresis platelet biology and physiology and the role of added, exogenous sex hormones. With addition to platelets, estradiol is rapidly converted to estrone, and although little is known about testosterone, we sought to investigate if platelet activity and physiology were affected by these hormonal ligands occupying their respective receptors on the platelet cell surface.7 This study 1) characterizes the differences in baseline platelet function and metabolism in vitro conferred by the age and sex of the donor, 2) evaluates the effects of sex hormones on in vitro platelet function and platelet metabolism, and 3) expands upon the limited knowledge of apheresis platelets. We hypothesize that testosterone administration inhibits the function of apheresis platelets and that there are baseline differences in platelet function and metabolism that may be stratified by sex and age.
Methods
Materials.
Testosterone, estradiol, and platelet-activating factor (PAF) were purchased from Sigma-Aldrich, Inc (St. Louis, MO). All reagents for the extent of shape change and aggregation experiments, including adenosine diphosphate (ADP), and thrombin were purchased from Chrono-Log Corporation (Haverton, PA). Antibodies for flow cytometry were purchased from BD Biosciences (Franklin Lakes, NJ): CD41a-FITC clone HIP8 and CD62-PE clone ACL-2. Seahorse reagents were purchased from Agilent Technologies (Santa Clara, CA). Source platelet poor plasma (PPP) was outdated plasma which had been separated from whole blood and drawn from OM and 2 OF donors. For experiments for which dilution with PPP was required, all platelet samples were diluted in the same source PPP for that experimental day such that all samples from their respective donor groups received the identical source PPP.
Apheresis platelets.
All platelets originated from intermediate altitude drawing centers, range 976–1848 meters above sea level: Denver, CO; Cheyenne, WY; Rapid City, SD, and were drawn on day 0 (the day of donation) using Terumo (Lakewood, CO) Trima apheresis machines with 100% being single apheresis units and stored or shipped to Vitalant-Denver. A sample from day 1 apheresis platelets, which were 14–22 hours post-draw with a median of 16 hours, from healthy donors were obtained under a Colorado Multiple Institute Review Board approved protocol (COMIRB#00–004), were not washed, and were grouped accordingly: 18–53 year-old premenopausal females, younger females (YF), ≥54 year-old, postmenopausal females, older females (OF), 18–44 year-old males, younger males (YM), ≥45 year-old males, older males (OM).8–16 The subdivision of females was based on the average age of menopause of ≥54 years and the gradual fall in total testosterone levels which begins around 45 years of age after peaking at 19 years and was verified by sex hormone levels.8–15 Platelet samples were naïve: no sex hormone/DMSO vehicle [0.0001%]FINAL or incubated with testosterone [5.31 ng/ml], the median physiologic level in healthy males >18 years), or estradiol [105 pg/ml], the median physiologic level in healthy premenopausal females during mid-estrus, for 15 minutes at 37°C.4,13–15,17–19
Extent of Shape Change.
Platelet function was assessed by extent shape change using light aggregometry (Chrono-Log Model 490), as described.20 Platelets were diluted in source PPP to 300×106 platelets/ml, warmed to 37°C, incubated with 1 mM EDTA, and shape change was measured in response to buffer, 20 μM ADP or 2 μM PAF.
Aggregation and ELISA Assays.
Aggregation was measured by light transmission (Chrono-Log Model 700) using 300×106 platelets/ml diluted in source PPP in response to the following: buffer (control), 20 μM of ADP, 2 μM of PAF, or 0.3 U/ml thrombin. Aggregation was calculated as the % Maximum Amplitude (Maximum Aggregation; % MA) from baseline. Human Vascular Endothelial Growth Factor (VEGF), testosterone, and estradiol were measured via a commercial ELISAs (VEGF: R&D, Minneapolis, MN or ABCAM (Boston, MA)) in the supernatants of platelets for VEGF and sex hormones.21
Flow Cytometry.
Platelets were diluted to a concentration of 6×104 platelets/ml and stimulated with buffer, 20 μM ADP for 5 minutes, or 2 μM PAF for 10 minutes and fixed with 1% paraformaldehyde. The fixed platelets were incubated with a FITC or PE-labeled isotype IgG control, or an antibody specific for CD41a (fibrinogen receptor, GPIIb) or P-selectin (CD62P) to assess platelet activation. The amount of CD41a or CD62P surface expression was measured using a BD FACSCanto II flow cytometer and CD41a mean fluorescent intensity (MFI) and % CD62P were calculated.
Mitochondrial Stress Test.
Apheresis platelets were incubated with Prostaglandin I2 (PgI2) [1 ng/ml] (Cayman Chemical Company, Ann Arbor, MI) to inhibit aggregation, centrifuged at 1000g for 5 minutes, resuspended in Tyrode’s Buffer, and diluted to a final concentration of 300×106 platelets/ml. 22×106 platelets plus Seahorse media for a final volume of 100 μl were loaded onto the sensor cartridge for the Seahorse XFe24 Analyzer. To measure mitochondrial metabolism the Seahorse XF Cell Mito Stress Kit (Agilent) was used to assess oxygen consumption and pH changes. After the initial baseline measurement, either hormone (testosterone or estradiol diluted into seahorse media) or vehicle control (seahorse media/0.0001% DMSO) was injected. This was followed by sequential addition of 1.5 μM Oligomycin, 1.0 μM Carbonyl cyanide-4 phenylhydrazone (FCCP), and 0.5 μM Rotenone/Antimycin for oxygen and pH measurements, per assay protocol. The oxygen consumption rate (OCR) was calculated in pmol/min for Basal Respiration, ATP-linked Respiration, Maximal Respiration, Spare Respiratory Capacity, and Nonmitochondrial Respiration. Extracellular Acidification Rate (ECAR) was calculated in mpH/min for non-sensitive/basal and oligomycin sensitive.
Statistics.
Statistical analysis was completed using GraphPad Prism Software V9 (San Diego, CA). Intragroup data from platelets treated with sex hormones were compared via paired (repeated measures), two-way analysis of variance (ANOVA) followed by Holm-Sidek test for multiple comparisons, based upon the equality of variance. Intergroup data, from platelets isolated from males or females stratified by age were analyzed by an independent (unpaired), two-way ANOVA between donor groups again followed by Holm-Sidek test for multiple comparisons. Significance equaled p<0.05.
Results
Demographics.
In total, 265 healthy donors comprised this study and were needed to ensure the sample size for each individual experiments because the sample size was only 3 ml and not all experiments could be completed for each individual donor: 57 YF, 19–44 years (mean 37.4), 68 postmenopausal OF, 55–76 years (mean 63.7), 59 YM, 18–44 years (mean 32.6) and 81 OM, 45–91 years (mean 62.3) (Table 1). Blood type A (51.4%) was most common followed by type O (29.5%), type B (9.9%) and type AB (9.5%) (Table 1). The overwhelming majority (90.7%) were Rhesus factor positive (Table 1). Estradiol and free testosterone levels were measured in EDTA plasma from selected donors solely from Denver, CO because estrogen measurements are affected by citrate and estradiol may be concentrated due to platelet release separating the PPP fraction of apheresis platelets.22–24 Both the OF and OM had less estradiol and free testosterone versus YF and YM, respectively, though not statistically different (Table 1).
Table 1:
Donor Demographics, VEGF Release, and Sex Hormone Levels
| Sex | Age groups | Mean Age years | Total Donors (265) | Blood Type (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | O | AB | ||||||||
| + | − | + | − | + | − | + | − | ||||
| Female | Younger | 37.4 (18–53) | 57 | 11.7 | 0.4 | 1.9 | 0.8 | 3.8 | 1.1 | 1.9 | 0.0 |
| Older | 63.7 (55–76) | 68 | 13.6 | 0.8 | 3.0 | 0.4 | 6.0 | 0.4 | 1.5 | 0.0 | |
| Male | Younger | 32.6 (19–44) | 59 | 10.6 | 0.4 | 2.3 | 0.0 | 6.1 | 0.4 | 1.9 | 0.8 |
| Older | 62.2 (45–91) | 81 | 12.8 | 1.1 | 1.1 | 0.4 | 10.6 | 1.1 | 1.9 | 1.5 | |
| Female (n=10 each group) | Naïve | Control | 52.3±3.4 | Estradiol (pg/ml) NL 28–549 (Pre-menopausal) | YF | 161.4±49.8 |
| ADP | 50.4±2.9 | |||||
| PAF | 59.9±5.3 | |||||
| Testosterone | Control | 45.4±1.8 | OF | 121.7±41.9 | ||
| ADP | 57.5±3.5* | |||||
| PAF | 51.7±2.3 | |||||
| Male (n=10 each group) | Naïve | Control | 56.3±5.0 | Free Testosterone (pg/ml) NL 4.5–42 | YM | 7.1±2.0 |
| ADP | 60.8±6.0 | |||||
| PAF | 70.0±5.8* | |||||
| Testosterone | Control | 53.6±4.2 | OM | 5.5±0.9 | ||
| ADP | 65.6±7.0* | |||||
| PAF | 60.1±5.7† |
p<0.05 from Control,
p<0.05 from Naïve (no sex hormone/DMSO vehicle)
ESC.
Platelets (300×106/ml) were naïve (no sex hormone/[0.0001%]FINAL DMSO) or pre-treated with testosterone for 15 minutes, and ESC was measured in response to either 20 μM ADP or 2 μM PAF. There were no differences in the %ESC of the platelets between either males or females when pre-treated with testosterone and stimulated with ADP or PAF (data not shown).
Platelet Aggregation.
Platelets (300×106/ml) were naïve or pre-treated with either estradiol or testosterone, and platelet aggregation was measured in response to various mediators. Testosterone incubation significantly decreased aggregation vs. naïve (no sex hormone pre-incubation) in OF and OM for ADP-mediated aggregation and in all donor groups for PAF-mediated aggregation (Fig.1A&B, p<0.05). Estradiol pre-incubation did decrease ADP-mediated platelet aggregation in OM but did not affect any of the other patient cohorts in response to ADP (Fig.1D).
Figure 1. The effects of testosterone- and estradiol- pre-treatments on the aggregation profile of apheresis platelets vs. naïve (0.0001% DMSO/no sex hormone) NS-treated platelets, compared within and between sex and age groups.
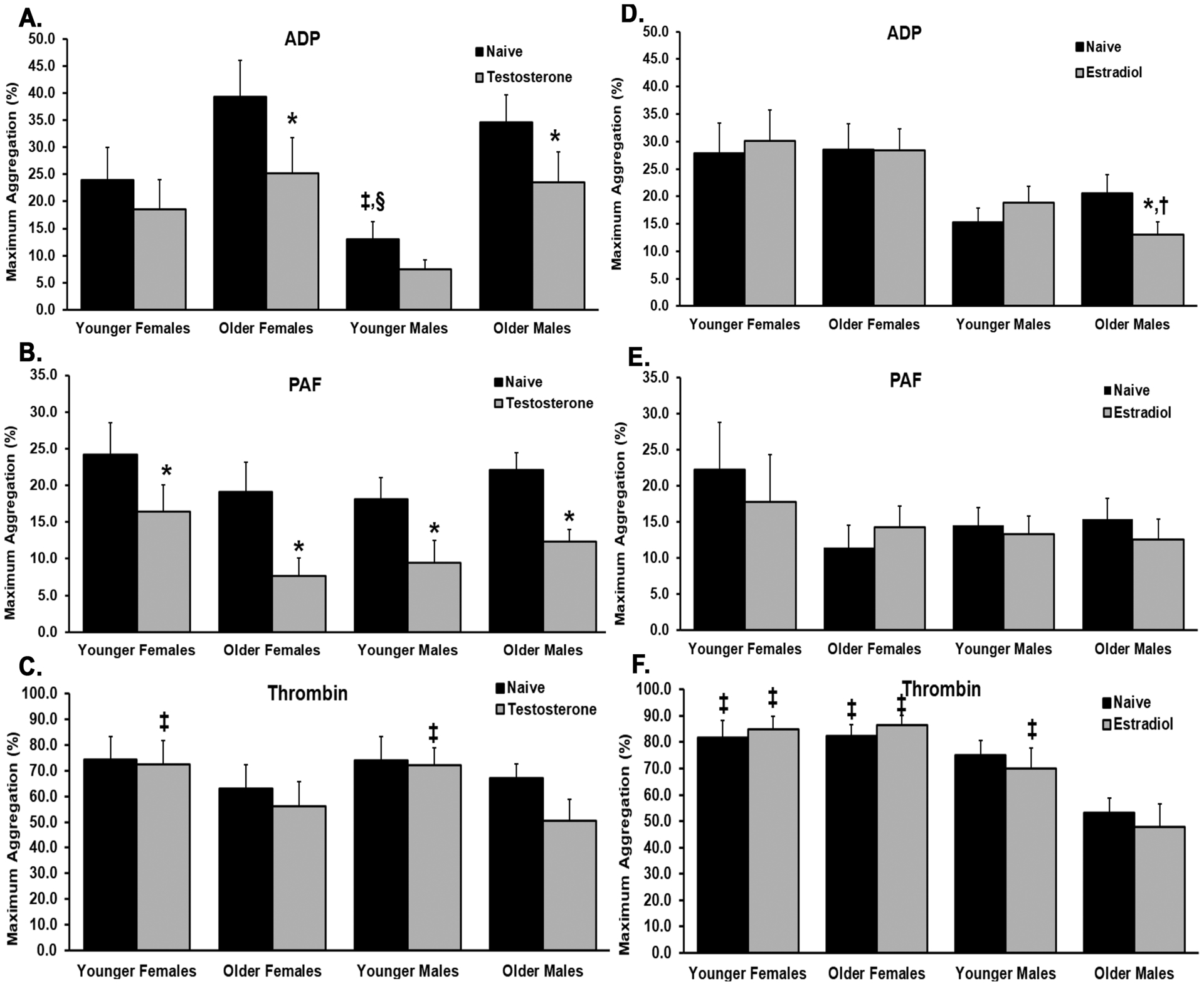
Platelet aggregations were measured in response to ADP, PAF, or thrombin and only the significant values are mentioned: Panel A) ADP: naïve OF, OM vs. testosterone-treated platelet aggregation, naïve YM vs. OF, older males, Panel B) PAF: testosterone-pretreated platelets vs naïve in all groups, Panel C) Thrombin: no significance, Panel D) ADP: naïve OM vs. estradiol-treated, OM estradiol-pretreated platelets vs. estradiol-treated platelets from YF, Panel E) PAF: No significance, Panel F) Thrombin: testosterone-treated OM from YF and YM; naïve OM vs. naïve YF and OF, estradiol-treated OM vs. all estradiol groups. Designation of symbols: * versus naïve NS-treated, † versus YF, ‡ versus OM, § versus OF, p<0.05 (n=10 in all groups)
Naïve platelets from YM had significantly decreased ADP-mediated aggregation versus naïve platelets from OM and OF, and estradiol-treated platelets had decreased aggregation to ADP versus estradiol-treated platelets from YF (Fig.1A&D, p<0.05, respectively). Thrombin-induced aggregation in testosterone-treated platelets from YF and YM was significantly increased from OM (Fig. 1C, p<0.05). Thrombin-induced aggregation in the platelets from YF, and OF were significantly increased versus OM naïve and in YF, OF, and YM estradiol-treated platelets (Fig.1F, p<0.05, respectively).
CD62P and CD41a Surface Expression.
In all groups, ADP and PAF caused significant platelet activation based on increased CD62P (%) surface expression. Naïve and testosterone-treated YF platelets had significantly lower percentage of CD62P than naïve platelets from YM and testosterone-treated platelets from YM and OF (Fig.2A). Similar to CD62P surface expression, ADP and PAF stimulation significantly increased CD41a (MFI) (Fig.2B). Estradiol pre-treatment in OF and OM control platelets had a significant increase in CD41a from the paired naïve platelets; testosterone pre-treatment in control YM and OM platelets was also significantly increased from paired naïve platelets. ADP stimulated estradiol treated platelets in OF and OM was significantly higher than naïve, ADP stimulated platelets (Fig.2B).
Figure 2. The effects of estradiol and testosterone pretreatment on the activation (CD62P expression) and surface expression of CD41a.
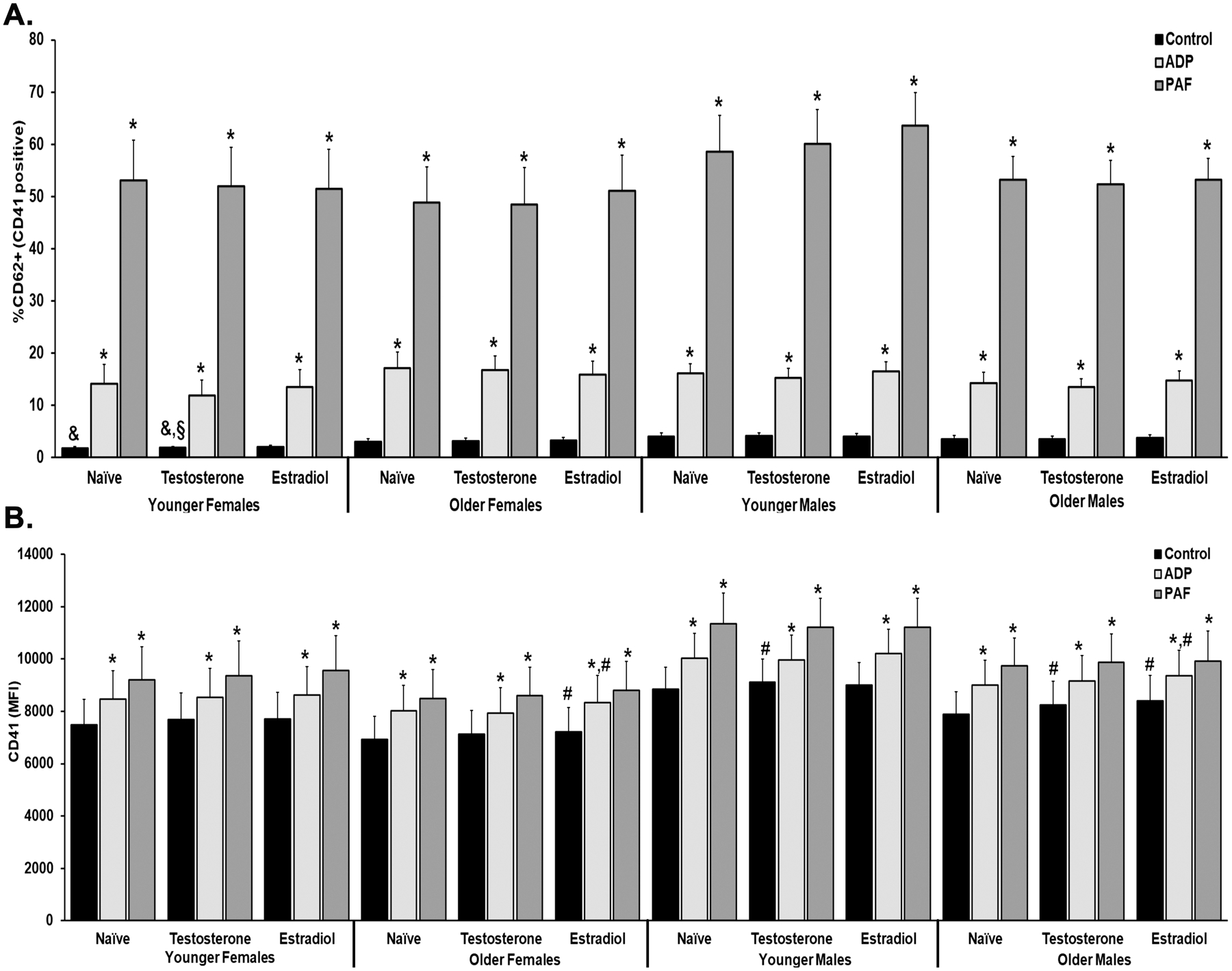
The % CD62P expression from CD41a+ platelets were measured by flow cytometry in apheresis platelets pre-treated with buffer (naïve), testosterone, or estradiol and the stimulated with NS (control), ADP, or PAF. A. The % CD62P was significantly increased in all stimulated (ADP and PAF) platelets. The control (unstimulated) platelets from YF, both naïve and testosterone-treated had significantly lower % CD62P expression than YM and OF (testosterone only). B. CD41a surface expression (MFI) was measured and ADP and PAF caused significant increases from control in naïve, testosterone, and estradiol pretreated groups. In YM and OM testosterone pre-treatment increased CD41a surface expression vs. the naïve. Estradiol pre-treatment increased the CD41a MFI in OF and OM naïve and ADP-stimulated platelets. Designation of symbols: * versus control; # versus Naïve, p<0.05 (n=10–11)
VEGF Release.
In naïve male platelets PAF significantly increased VEGF release (p<0.05) (Table 1). ADP significantly increased VEGF release in both male and female platelets pre-treated with testosterone versus naïve platelet controls (p<0.05). Lastly, testosterone pretreatment of male platelets significantly inhibited PAF-mediated VEGF release vs. naïve controls (Table 1, p<0.05).
Mitochondrial Stress Test.
Figure 3 depicts the mean ± SEM of the OCR for 10 subjects in each sex/age group stratified by pretreatment: vehicle (Panel A), testosterone (Panel B) or estradiol (Panel C) (Fig.3). Figure 4 is comprised of 6 individual measurements from the data in Fig. 3.
Figure 3. A compilation of mitochondrial respiration in platelets from donors stratified by sex and age.
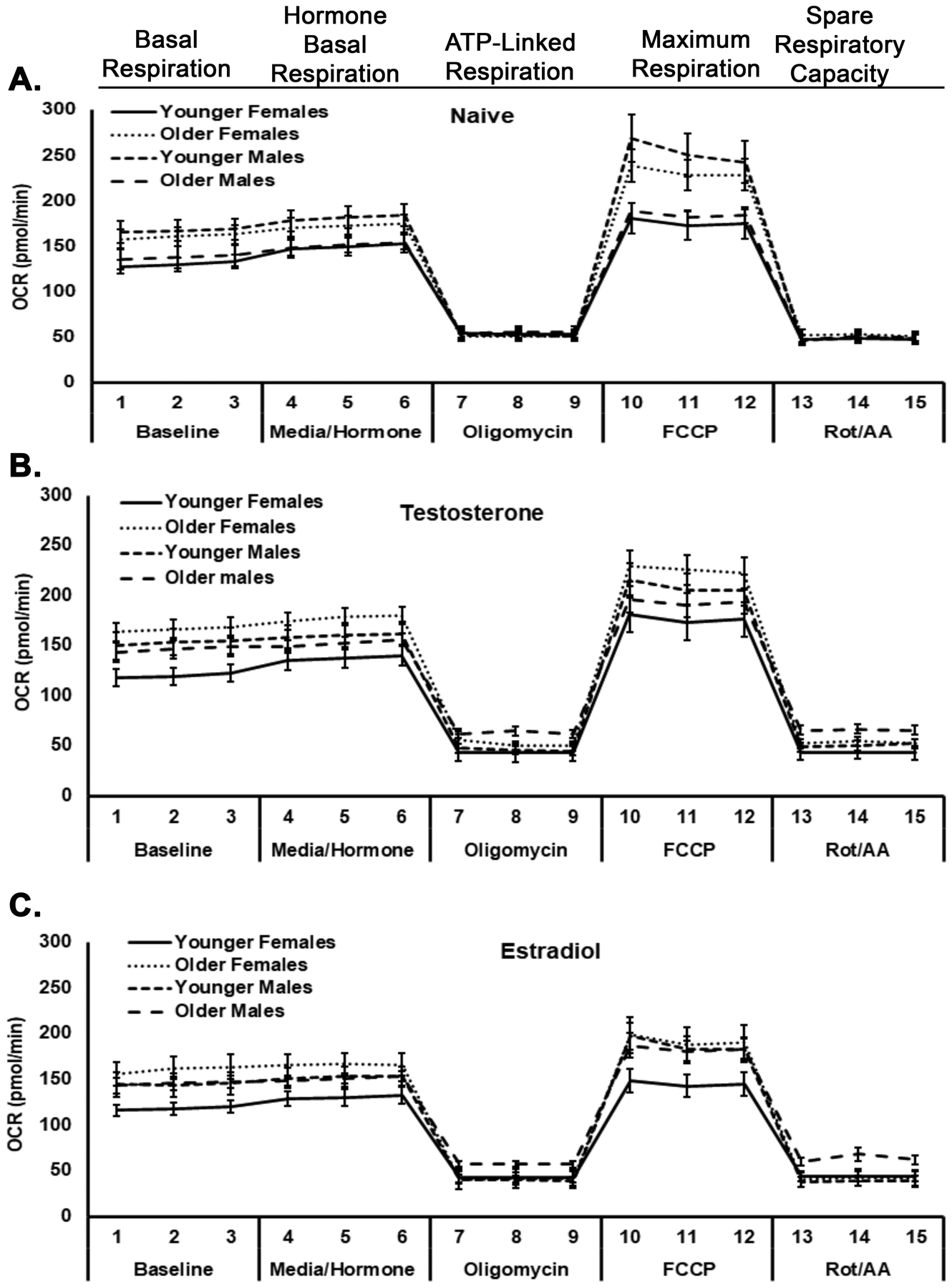
The mitochondrial respiration in platelets from YF, OF, YM, and OM are illustrated in Seahorse tracings from naïve (media-treated) platelets (Panel A), testosterone-treated platelets (Panel B), and estradiol-treated platelets (Panel C). Oxygen consumption rate (OCR, mean±SEM) of platelets from different ages/sexes is shown as a function of different sequential measurements of mitochondrial stress treatment: baseline and hormone/vehicle additions (basal respiration), oligomycin (ATP-linked respiration), FCCP (maximum respiration) and Rot/AA (spare respiratory capacity); n=10 for each group.
Figure 4. Platelet respiration stratified by donor sex and age.
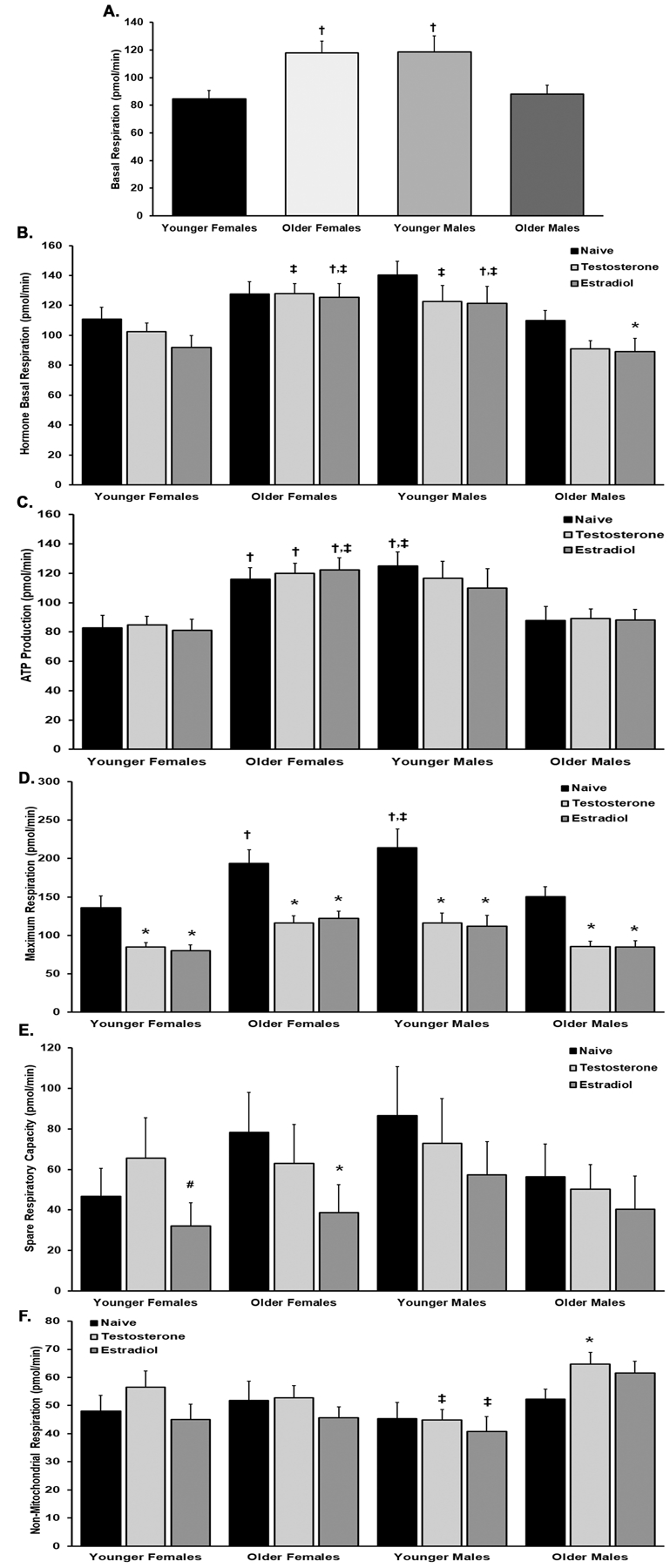
Panel A The basal respiration of platelets from YF was significantly less than the basal respiration of platelets from both OF and YM (p<0.05) (). Panel B. Estradiol pretreatment significantly decreased the basal respiration of platelets from OM (p<0.05). Testosterone pretreatment increased the basal respiration in OF and YM vs OM and estradiol pre-treatment increased the basal respiration of platelets in both OF and YM compared to younger females and older males (p<0.05). Panel C. ATP-linked production was significantly higher in older females in naïve, testosterone- and estradiol-treated platelets vs YF but only estradiol was significant from OMs. YM naïve platelets were significant from both YF and OM. Panel D. Hormone treatment caused a significant decrease amongst all groups for the maximum respiration. The maximum respiration of naïve platelets from OF and YM was significantly higher than YF, but only YM were statistically different from OM. Panel E. The spare respiratory capacity was only affected by estradiol treatment in YF and OF from naïve. Panel F. The non-mitochondrial respiration is significantly lower in YM versus the platelets from OM with hormone treatment, both testosterone and estradiol. Testosterone also caused a significant increase from naïve in the platelets from OM. Designation of symbols: * versus naïve, † versus YF, and ‡ versus OM, p<0.05 (n=10 in all groups).
The basal respiration (BR) of naïve platelets (buffer and media only) from both OF and YM was significantly increased when compared to the BR from platelets drawn from YF (Fig.4A). Estradiol incubation decreased the BR of platelets from OM versus controls, which did not occur in the other cohorts (Fig.4B, p<0.05). When comparing the BR between the age/sex groups both the estradiol and testosterone pre-treated platelets from OF and YM were increased versus estradiol- and testosterone-treated platelets from OM (Fig.4B, p<0.05). The BR from estradiol-pretreatment of platelets from OF and YM was significantly increased versus the BR from estradiol-pretreated platelets from YF (Fig.4B, p<0.05).
Although hormone treatment did not change ATP production compared to naïve platelets (Fig.4C), when comparing amongst the groups, ATP production was increased in naïve, testosterone- and estradiol-pretreated platelets in OF as compared to the naïve, testosterone-, and estradiol-pre-treated platelets from YF, respectively (Fig.4C, p<0.05), ATP production from naïve platelets from YM was also increased compared to ATP production in naïve platelets from both YF and OM; ATP production from estradiol-preincubated platelets OF was increased versus ATP production in estradiol-pretreated platelets from OM (Fig.4C, p<0.05).
Hormone treatment of platelets significantly decreased the maximum respiration (MR; maximum amount of oxygen consumption) versus naïve platelets, (Fig.4D). Both testosterone and estradiol pre-incubation significantly decreased the maximum respiration of platelets across all sex and age groups versus the respective naïve controls for each group (Fig.4D). When the groups stratified by age and sex were compared, the naïve platelets from YF have significantly less MR compared to naïve platelets from both OF and YM (p<0.05, respectively). Moreover, the MR of naïve platelets from younger males have significantly more MR versus the naïve platelets from older males (p<0.05) (Fig.4D).
The spare respiratory capacity (SRC), or “potential energy” that is reserved for platelet use during an energetic demand, were significantly affected by estradiol, since estradiol-treated platelets from both YF and OF had a significantly decreased SRC versus their respective naïve controls (Fig,4E, p<0.05).
Testosterone pretreatment increased the nonmitochondrial response (NMR) in the platelets from OM versus naïve controls (Fig.4F, p<0.05). When comparing between the groups, there were significant differences in NMR between both testosterone- and estradiol-treated platelets from younger males versus testosterone- and estradiol-treated platelets from OM (Fig.4F, p<0.05, respectively).
Although there were no intragroup differences in the extracellular acidification rate (ECAR) amongst naïve platelets versus sex hormone-pretreatment in the basal or the oligomycin sensitive tests (Figs.5A & B), when the groups stratified by age and sex were compared, there were multiple differences in the basal ECAR: naïve platelets from the OF (all treatment groups) had significantly more basal ECAR compared to naïve platelets from younger females and OM (p<0.05, respectively) (Fig.5C). There are even more differences between the groups in the oligomycin-sensitive ECAR: 1) estradiol-treated platelets have increased ECAR versus naïve controls from OF, 2) the platelets from OF have significantly more oligomycin-sensitive ECAR for all treatments (naïve, estradiol, and testosterone), versus platelets from YF and from OM (Fig.5B, p<0.05, respectively. The naïve platelets from YM had more oligomycin-sensitive ECAR versus both naïve platelets from both YF and OM (Fig.5B, p<0.05, respectively).
Figure 5. The platelet extracellular acidification rate (ECAR).
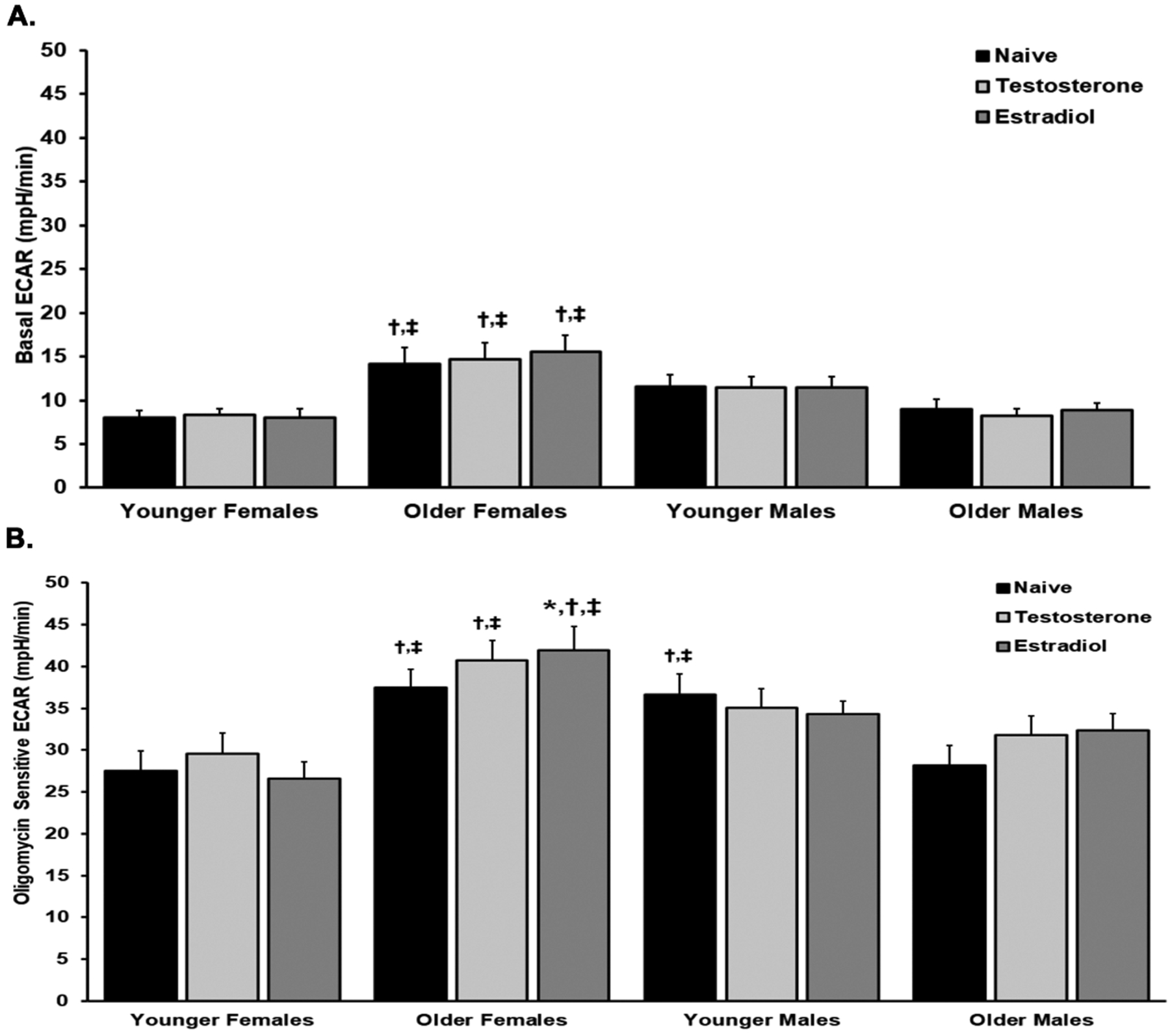
Pre-incubation with testosterone or estradiol did not affect the ECAR within the age/sex groups (Panel A). The naïve, testosterone- and estradiol-treated platelets from OF had significantly increased ECARs from YF and OM, respectively (p<0.05) (Panel A). Oligomycin-sensitive ECAR in platelets from OF was significantly increased from the platelet from both YF and OM in naïve, testosterone- and estradiol-treated platelets, and testosterone treatment also had a significant increase from naïve platelets (Panel B). The naïve platelets from YM were also significantly higher than the platelets from YF and OM (Panel B). Designation of symbols: * versus naïve, † versus YF, ‡ versus OM, p<0.05 (n=10 in all groups).
Discussion
The presented data comprises an extensive assessment on the function and metabolism of apheresis platelets with notable differences when stratified by donor sex and age, and the administration of exogenous sex hormones at physiologic levels. Previous data showed that apheresis platelets from female donors had increased ESC with ADP stimulation versus male platelets and as presented, testosterone did not affect the ESC in platelets from both female and male donors.4 Female platelets had increased aggregation versus male platelets, and more precisely, platelets from OF had more ADP-induced aggregation compared to YM; whereas, platelets from YF had increased thrombin-mediated aggregation versus platelets from both YM and OM. Additionally, exogenous testosterone administration decreased ADP-mediated aggregation in female platelets, irrespective of age and in platelets from OM in response to thrombin, but there were no intragroup differences when platelets were pretreated with estradiol. Estradiol pretreatment did increase thrombin-induced aggregation of platelets from YF, OF, and YM males versus platelets from OM.
In all donor groups both ADP and PAF increased CD62P surface expression versus the naïve controls. There were a few differences amongst the buffer-treated naïve and testosterone pre-treated platelets, which were small though significant. Increased surface expression of CD41a is important because it is the GPIIb segment of the glycoprotein IIb/IIIa (GPIIb/IIIa) receptor, with CD61 being the GPIIIa segment, and is a marker of global fibrinogen receptor surface expression.25–33 In its active form, the GPIIb/IIIa receptor is an important mediator for platelet aggregation by binding to fibrinogen and/or von Willebrand factor to cross-link platelets for stable thrombus formation.25–27,34 Similar to aggregation, testosterone pretreatment decreased CD41a surface expression in PAF-induced platelet activation in the platelets from YF, YM, and OM groups and similar CD41a data for estradiol-treated platelets has been reported.4
Platelet degranulation experiments illustrated that testosterone inhibited PAF-induced VEGF release from male platelets. Interestingly, this testosterone-mediated inhibition of VEGF release appears contrary to previous data which concluded that VEGF is increased with excess androgens in males with prostate cancer; however, the source of the VEGF in these patients may not be platelet-derived.35
This study also evaluated multiple components of platelet mitochondrial function related to sex, age, and exogenous hormone administration. Because platelets lack nuclei, the “relative platelet health” is dependent on mitochondrial function. Healthy platelets contain 5–8 mitochondria that are responsible for platelet metabolism, activation, and ATP production.36,37 Platelet energy demand is met with a complex system of glycolysis (60% ATP) and mitochondrial oxidative phosphorylation (40% ATP).37,38 The platelet is able to adapt to different environments via its metabolic flexibility.39 There are metabolomic differences in platelets dependent upon age and sex, notably that OM have increased levels of metabolites from the pentose phosphate pathway, Krebs cycle, amino acid metabolism, and free fatty acids.40 The presented data reflect these observations, but are variable given the microenvironment because there is a complex interplay of sex, age, and hormones, all of which may affect mitochondrial function. Both estradiol and testosterone increased the BR in certain groups as denoted previously. Secondly, the mitochondrial response to stress, ATP production, was different depending on sex and age in that naïve platelets from YF produced less ATP compared to all other groups while platelets from OM had less ATP production versus platelets from YM. Hormone pretreatment decreased ATP production in select groups. Thirdly for maximum respiration, preincubation with either testosterone or estradiol decreased the MR in the platelets from all groups compared to naïve controls; however, at baseline there are differences between the groups with the platelets from YF having less MR versus the platelets from OF and OM, and the platelets from OM have less MR than the platelets from YM.
In terms of the non-mitochondrial respiration capabilities, testosterone-preincubated platelets from OM have an increased NMR compared to the respective naïve controls group. Thus, the addition of testosterone to the platelets from relatively testosterone-depleted OM switches the cells’ respiration to be more dependent on pro-oxidant and pro-inflammatory enzymes, including cyclooxygenase, cytochrome P450, or NADPH oxidases.41 Interestingly, low-testosterone states, not high states, are associated with an increase in pro-inflammatory cytokines.42 Perhaps these data reflect the cellular inability to react to an acute rise in testosterone levels, which could be similar to the testosterone-induced inhibition of VEGF release. These results further highlight how blood donor sex and age could play a role on recipient physiology. Additional studies need to be performed to further assesses the mechanistic driver behind the mitochondrial stress response to sex hormone administration.
The mechanisms behind the differences imparted by donor sex dimorphisms and age have not been elucidated. In regards to the effect of sex on platelet function, in some studies males had decreased platelet aggregation versus females while others have shown the opposite.4,43–45 Enhanced platelet activity may occur in the elderly compared to younger individuals in relation to cardiovascular disease, and in these studies platelet activation and aggregation were more robust in the elderly versus than in younger patients.46–51,46,47,52,53 This enhanced platelet activity was hypothesized to be responsible for increased vascular and thrombotic pathology, leading to hypercoagulability and cardiovascular disease.46–51,46,47,52,53 In addition, with increased age, there is a significant decline in platelet number; in addition, females to have more platelets than males.54–58 To correct for these numerical differences, platelets were diluted to a standard concentration for all experiments to obviate any differences in platelet activity due to platelet number. Importantly, day 1 apheresis platelets are stored in plasma, were employed without additional washing, which may increase baseline platelet activity. Although the age stratification may appear indiscriminate, the differences in platelet function were apparent in the oldest in the YF and YM groups versus the youngest in the OM and OF groups and though both estradiol and testosterone were lower in OF and OM versus YF and YM, respectively they were not significantly different. Lastly, many studies employ platelets isolated from whole blood, and the reported data probes the question on which type of studies are most important for assessing platelet activity and function, those from platelets that cannot be “biologically used” or those platelets that will be transfused?
Sex and age of blood donors are not typically considered regarding platelet transfusions and supplies of platelet concentrates may be limited. As mentioned, there are significant differences in the circulating metabolites in the platelets from OM versus platelets from females and YM.40 However, female platelets have higher circulating glutamine concentrations and decreased levels of reduced glutathione, and decreased deaminated purines and arginine metabolism into polyamines.40 This study, amongst others, further exemplifies the differences in platelet activity depending on the donor age/sex, and the possible clinical impact that this may impart on platelet recipients. Lastly, although the addition of exogenous testosterone and estradiol does change platelet physiology, it does not explain the age/sex differences in platelet function. The 15 minutes of sex hormone incubation does result in receptor activation and non-nuclear changes in platelet physiology; longer incubations at higher concentrations may be warranted, though they still may not mimic years of sex hormone exposure on platelet progenitors, and animal modeling may be needed.7
This investigation focused on two sex hormones, and future investigations should be expanded to include progesterone and dehydroepiandrosterone. Platelet donors are not rejected for estrogen or testosterone supplementation and measuring these hormones in the plasma from apheresis platelets is not wholly accurate.22,59–61 Additionally, all platelets were drawn at donation centers where donor race is not screened, despite the possible influence of race on platelet physiology.62–64 Additional studies should focus on mechanistic drivers in hormone response and platelet function at molecular and cellular levels.
In conclusion, platelet function and metabolism differ based on the sex and age of the donor. Additionally, exogenous hormone administration at physiologic concentrations can affect platelet function, notably the inhibitory capacity of testosterone. Sex differences in platelet biology, because of baseline hormone status, question whether donor sex should be considered for transfusion of blood products, and although the overall differences are relatively small, they may represent a future avenue of studies for precision medicine.
Funding:
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health under grants T32GM008315 and RM1GM131968 and a grant UM1HL120877 from NHLBI, NIH and Vitalant Research Institute.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016;16: 626–38. [DOI] [PubMed] [Google Scholar]
- 2.Lee JW, Profant M, Wang C. Metabolic Sex Dimorphism of the Brain at the Gene, Cell, and Tissue Level. J Immunol 2022;208: 212–20. [DOI] [PubMed] [Google Scholar]
- 3.Jaillon S, Berthenet K, Garlanda C. Sexual Dimorphism in Innate Immunity. Clin Rev Allergy Immunol 2019;56: 308–21. [DOI] [PubMed] [Google Scholar]
- 4.Coleman JR, Moore EE, Kelher MR, Samuels JM, Cohen MJ, Sauaia A, Banerjee A, Silliman CC, Peltz ED. Female platelets have distinct functional activity compared with male platelets: Implications in transfusion practice and treatment of trauma-induced coagulopathy. The journal of trauma and acute care surgery 2019;87: 1052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gee AC, Sawai RS, Differding J, Muller P, Underwood S, Schreiber MA. The influence of sex hormones on coagulation and inflammation in the trauma patient. Shock 2008;29: 334–41. [DOI] [PubMed] [Google Scholar]
- 6.Khetawat G, Faraday N, Nealen ML, Vijayan KV, Bolton E, Noga SJ, Bray PF. Human megakaryocytes and platelets contain the estrogen receptor beta and androgen receptor (AR): testosterone regulates AR expression. Blood 2000;95: 2289–96. [PubMed] [Google Scholar]
- 7.Plotka ED, Nikolai TF, Hague SS. The interaction between estradiol and human platelets. Clin Chim Acta 1973;49: 287–93. [DOI] [PubMed] [Google Scholar]
- 8.Kelsey TW, Li LQ, Mitchell RT, Whelan A, Anderson RA, Wallace WHB. A validated age-related normative model for male total testosterone shows increasing variance but no decline after age 40 years. PloS one 2014;9: e109346–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morley JE, Perry HM 3rd. Androgen deficiency in aging men: role of testosterone replacement therapy. J Lab Clin Med 2000;135: 370–8. [DOI] [PubMed] [Google Scholar]
- 10.Morley JE, Unterman TG. Hormonal fountains of youth. J Lab Clin Med 2000;135: 364–6. [DOI] [PubMed] [Google Scholar]
- 11.Stanworth RD, Jones TH. Testosterone for the aging male; current evidence and recommended practice. Clinical interventions in aging 2008;3: 25–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellison PT, Bribiescas RG, Bentley GR, Campbell BC, Lipson SF, Panter-Brick C, Hill K. Population variation in age-related decline in male salivary testosterone. Hum Reprod 2002;17: 3251–3. [DOI] [PubMed] [Google Scholar]
- 13.Diver M Laboratory measurement of testosterone. Front Horm Res 2009;37: 21–31. [DOI] [PubMed] [Google Scholar]
- 14.Diver MJ. Analytical and physiological factors affecting the interpretation of serum testosterone concentration in men. Ann Clin Biochem 2006;43: 3–12. [DOI] [PubMed] [Google Scholar]
- 15.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas 2008;61: 4–16. [DOI] [PubMed] [Google Scholar]
- 16.ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol 2014;123: 202–16. [DOI] [PubMed] [Google Scholar]
- 17.Laboratories MC. Testosterone, Total, Bioavailable, and Free, Serum [monograph on the internet]. MayoAccess Mayo Clinic Laboratories. Available from: https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/83686 [Google Scholar]
- 18.Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A Reappraisal of Testosterone’s Binding in Circulation: Physiological and Clinical Implications. Endocr Rev 2017;38: 302–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA, Lightner DJ, Miner MM, Murad MH, Nelson CJ, Platz EA, Ramanathan LV, Lewis RW. Evaluation and Management of Testosterone Deficiency: AUA Guideline. J Urol 2018;200: 423–32. [DOI] [PubMed] [Google Scholar]
- 20.Holme S, Moroff G, Murphy S. A multi-laboratory evaluation of in vitro platelet assays: the tests for extent of shape change and response to hypotonic shock. Biomedical Excellence for Safer Transfusion Working Party of the International Society of Blood Transfusion. Transfusion 1998;38: 31–40. [DOI] [PubMed] [Google Scholar]
- 21.Kanter J, Khan SY, Kelher M, Gore L, Silliman CC. Oncogenic and angiogenic growth factors accumulate during routine storage of apheresis platelet concentrates. Clin Cancer Res 2008;14: 3942–7. [DOI] [PubMed] [Google Scholar]
- 22.Denver N, Khan S, Homer NZM, MacLean MR, Andrew R. Current strategies for quantification of estrogens in clinical research. J Steroid Biochem Mol Biol 2019;192: 105373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dilks JR, Flaumenhaft R. Fluoxetine (Prozac) augments platelet activation mediated through protease-activated receptors. J Thromb Haemost 2008;6: 705–8. [DOI] [PubMed] [Google Scholar]
- 24.Sarabia SF, Raya JL, Hoogeveen RC, Bray PF. Estradiol is concentrated in human platelets and is released upon activation. Blood 2006;108: 3916.16902145 [Google Scholar]
- 25.Yun SH, Sim EH, Goh RY, Park JI, Han JY. Platelet Activation: The Mechanisms and Potential Biomarkers. Biomed Res Int 2016;2016: 9060143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas MR, Storey RF. The role of platelets in inflammation. Thromb Haemost 2015;114: 449–58. [DOI] [PubMed] [Google Scholar]
- 27.Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost 2009;102: 248–57. [DOI] [PubMed] [Google Scholar]
- 28.Akay OM, Gunduz E, Basyigit H, Gulbas Z. Platelet function testing during 5-day storage of single and random donor plateletpheresis. Transfus Apher Sci 2007;36: 285–9. [DOI] [PubMed] [Google Scholar]
- 29.Aurigemma C, Fattorossi A, Sestito A, Sgueglia GA, Farnetti S, Buzzonetti A, Infusino F, Landolfi R, Scambia G, Crea F, Lanza GA. Relationship between changes in platelet reactivity and changes in platelet receptor expression induced by physical exercise. Thromb Res 2007;120: 901–9. [DOI] [PubMed] [Google Scholar]
- 30.Ballow A, Gader AM, Huraib S, Al-Husaini K, Mutwalli A, Al-Wakeel J. Platelet surface receptor activation in patients with chronic renal failure on hemodialysis, peritoneal dialysis and those with successful kidney transplantation. Platelets 2005;16: 19–24. [DOI] [PubMed] [Google Scholar]
- 31.Berger G, Masse JM, Cramer EM. Alpha-granule membrane mirrors the platelet plasma membrane and contains the glycoproteins Ib, IX, and V. Blood 1996;87: 1385–95. [PubMed] [Google Scholar]
- 32.Blair TA, Michelson AD, Frelinger AL 3rd. Mass Cytometry Reveals Distinct Platelet Subtypes in Healthy Subjects and Novel Alterations in Surface Glycoproteins in Glanzmann Thrombasthenia. Sci Rep 2018;8: 10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frelinger AL 3rd. Using flow cytometry to monitor glycoprotein IIb-IIIa activation. Platelets 2018;29: 670–6. [DOI] [PubMed] [Google Scholar]
- 34.Tardy B, Lecompte T, Mullier F, Vayne C, Pouplard C. Detection of Platelet-Activating Antibodies Associated with Heparin-Induced Thrombocytopenia. Journal of clinical medicine 2020;9: 1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eisermann K, Fraizer G. The Androgen Receptor and VEGF: Mechanisms of Androgen-Regulated Angiogenesis in Prostate Cancer. Cancers (Basel) 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi T, Tanaka S, Hori Y, Hirayama F, Sato EF, Inoue M. Role of mitochondria in the maintenance of platelet function during in vitro storage. Transfus Med 2011;21: 166–74. [DOI] [PubMed] [Google Scholar]
- 37.Melchinger H, Jain K, Tyagi T, Hwa J. Role of Platelet Mitochondria: Life in a Nucleus-Free Zone. Front Cardiovasc Med 2019;6: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Wu Q, Fan Z, Xie R, Wang Z, Lu Y. Platelet mitochondrial dysfunction and the correlation with human diseases. Biochem Soc Trans 2017;45: 1213–23. [DOI] [PubMed] [Google Scholar]
- 39.Doery JC, Hirsh J, Cooper I. Energy metabolism in human platelets: interrelationship between glycolysis and oxidative metabolism. Blood 1970;36: 159–68. [PubMed] [Google Scholar]
- 40.D’Alessandro A, Stefanoni D, Slichter SJ, Fu X, Zimring JC. The impact of donor sex and age on stored platelet metabolism and post-transfusion recovery. Blood Transfus 2021;19: 216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chacko Balu K, Kramer Philip A, Ravi S, Benavides Gloria A, Mitchell T, Dranka Brian P, Ferrick D, Singal Ashwani K, Ballinger Scott W, Bailey Shannon M, Hardy Robert W, Zhang J, Zhi D, Darley-Usmar Victor M. The Bioenergetic Health Index: a new concept in mitochondrial translational research. Clinical Science 2014;127: 367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohamad NV, Wong SK, Wan Hasan WN, Jolly JJ, Nur-Farhana MF, Ima-Nirwana S, Chin KY. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 2019;22: 129–40. [DOI] [PubMed] [Google Scholar]
- 43.Boudoulas KD, Montague CR, Goldschmidt-Clermont PJ, Cooke GE. Estradiol increases platelet aggregation in Pl(A1/A1) individuals. Am Heart J 2006;152: 136–9. [DOI] [PubMed] [Google Scholar]
- 44.Miller CH, Rice AS, Garrett K, Stein SF. Gender, race and diet affect platelet function tests in normal subjects, contributing to a high rate of abnormal results. Br J Haematol 2014;165: 842–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu GJ, Lee JJ, Chou DS, Jayakumar T, Hsiao G, Chen WF, Sheu JR. Inhibitory signaling of 17β-estradiol in platelet activation: the pivotal role of cyclic AMP-mediated nitric oxide synthase activation. Eur J Pharmacol 2010;649: 140–9. [DOI] [PubMed] [Google Scholar]
- 46.Le Blanc J, Lordkipanidzé M. Platelet Function in Aging. Front Cardiovasc Med 2019;6: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davizon-Castillo P, McMahon B, Aguila S, Bark D, Ashworth K, Allawzi A, Campbell RA, Montenont E, Nemkov T, D’Alessandro A, Clendenen N, Shih L, Sanders NA, Higa K, Cox A, Padilla-Romo Z, Hernandez G, Wartchow E, Trahan GD, Nozik-Grayck E, Jones K, Pietras EM, DeGregori J, Rondina MT, Di Paola J. TNF-α-driven inflammation and mitochondrial dysfunction define the platelet hyperreactivity of aging. Blood 2019;134: 727–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med 2007;357: 2482–94. [DOI] [PubMed] [Google Scholar]
- 49.Breddin HK, Lippold R, Bittner M, Kirchmaier CM, Krzywanek HJ, Michaelis J. Spontaneous platelet aggregation as a predictive risk factor for vascular occlusions in healthy volunteers? Results of the HAPARG Study. Haemostatic parameters as risk factors in healthy volunteers. Atherosclerosis 1999;144: 211–9. [DOI] [PubMed] [Google Scholar]
- 50.Jackson SP. Arterial thrombosis--insidious, unpredictable and deadly. Nat Med 2011;17: 1423–36. [DOI] [PubMed] [Google Scholar]
- 51.Puurunen MK, Hwang SJ, Larson MG, Vasan RS, O’Donnell CJ, Tofler G, Johnson AD. ADP Platelet Hyperreactivity Predicts Cardiovascular Disease in the FHS (Framingham Heart Study). J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gregg D, Goldschmidt-Clermont PJ. Cardiology patient page. Platelets and cardiovascular disease. Circulation 2003;108: e88–90. [DOI] [PubMed] [Google Scholar]
- 53.Liang W, Zhang P, Liu M. Association between renal function and platelet reactivity during aspirin therapy in elderly patients with atherosclerotic cardiovascular disease. BMC Geriatrics 2021;21: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segal JB, Moliterno AR. Platelet counts differ by sex, ethnicity, and age in the United States. Ann Epidemiol 2006;16: 123–30. [DOI] [PubMed] [Google Scholar]
- 55.Troussard X, Vol S, Cornet E, Bardet V, Couaillac JP, Fossat C, Luce JC, Maldonado E, Siguret V, Tichet J, Lantieri O, Corberand J. Full blood count normal reference values for adults in France. J Clin Pathol 2014;67: 341–4. [DOI] [PubMed] [Google Scholar]
- 56.Robbins J, Wahl P, Savage P, Enright P, Powe N, Lyles M. Hematological and biochemical laboratory values in older Cardiovascular Health Study participants. J Am Geriatr Soc 1995;43: 855–9. [DOI] [PubMed] [Google Scholar]
- 57.Emery JD, Leifer DW, Moura GL, Southern P, Morrissey JH, Lawrence JB. Whole-blood platelet aggregation predicts in vitro and in vivo primary hemostatic function in the elderly. Arterioscler Thromb Vasc Biol 1995;15: 748–53. [DOI] [PubMed] [Google Scholar]
- 58.Kasjanovová D, Baláz V. Age-related changes in human platelet function in vitro. Mech Ageing Dev 1986;37: 175–82. [DOI] [PubMed] [Google Scholar]
- 59.Kohek M, Leme C, Nakamura IT, De Oliveira SA, Lando V, Mendonca BB. Effects of EDTA and Sodium Citrate on hormone measurements by fluorometric (FIA) and immunofluorometric (IFMA) methods. BMC Clin Pathol 2002;2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosner W, Hankinson SE, Sluss PM, Vesper HW, Wierman ME. Challenges to the measurement of estradiol: an endocrine society position statement. J Clin Endocrinol Metab 2013;98: 1376–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vitku J, Chlupacova T, Sosvorova L, Hampl R, Hill M, Heracek J, Bicikova M, Starka L. Development and validation of LC-MS/MS method for quantification of bisphenol A and estrogens in human plasma and seminal fluid. Talanta 2015;140: 62–7. [DOI] [PubMed] [Google Scholar]
- 62.Tourdot BE, Conaway S, Niisuke K, Edelstein LC, Bray PF, Holinstat M. Mechanism of race-dependent platelet activation through the protease-activated receptor-4 and Gq signaling axis. Arterioscler Thromb Vasc Biol 2014;34: 2644–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mumaw MM, Nieman MT. Race differences in platelet reactivity: is protease activated receptor 4 a predictor of response to therapy? Arterioscler Thromb Vasc Biol 2014;34: 2524–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edelstein LC, Simon LM, Montoya RT, Holinstat M, Chen ES, Bergeron A, Kong X, Nagalla S, Mohandas N, Cohen DE, Dong JF, Shaw C, Bray PF. Racial differences in human platelet PAR4 reactivity reflect expression of PCTP and miR-376c. Nat Med 2013;19: 1609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]


