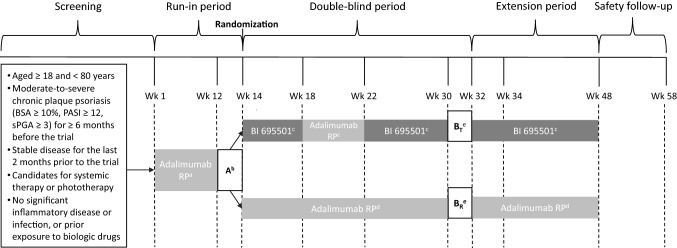
Fig. 1.
Study design. aAdalimumab RP 80 mg loading dose on Day 1 and then 40 mg/0.8 mL or 40 mg/0.4 mL EOW from Week 2 to Week 12. bFirst PK sampling interval (adalimumab RP only). cBI 695501 40 mg/0.8 mL at Week 14 and Week 16, adalimumab RP 40 mg/0.8 mL EOW at Week 18 and Week 20, and then BI 695501 40 mg/0.8 mL EOW from Week 22 to Week 48. dAdalimumab RP 40 mg/0.8 mL EOW from Week 14 to Week 48. ePrimary endpoints assessment period, and the second PK sampling interval (Week 30–32) in switching (BT) and continuous (BR) arms. BSA body surface area, EOW every other week, PASI psoriasis area and severity index, RP reference product, sPGA Static Physician’s Global Assessment