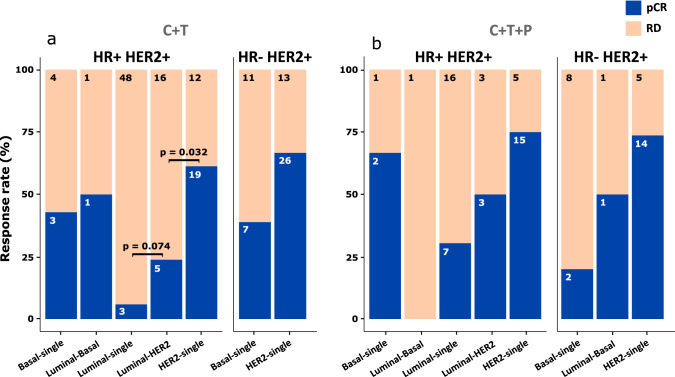
Fig. 5.
Distribution of pathologically confirmed HER2+ patients of the NBRST trial [18–20] based on the BluePrint single and dual subtype classification and their treatment response (N = 253). Patients are grouped based on their therapy regimen [chemotherapy (C) plus Trastuzumab (T) (panel a) or C + T and Pertuzumab (P) (panel b)], and their HR and HER2 status (HR+ HER2 + or HR- HER2+. The colored bars represents if a tumor did (pCR, blue) or did not [Residual Disease (RD), bisque] achieve pathological complete response (pCR). p-value determined with a chi-square test of independence between subtypes. Of the entire NBRST set (n = 289), 253 samples are showed due to low numerosity of HER2-Basal-type (n = 19) and Luminal-HER2-Basal (n = 17)