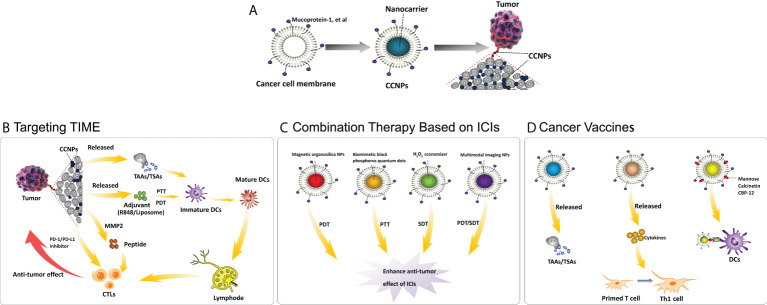
Figure 1.
Application of cancer cell membrane-wrapped nanoparticles for cancer immunotherapy. (A) The structure of CCNPs; (B) Mechanism of CCNPs targeting on TME: CCNPs directly carry TSAs from cancer cell membranes or by different stimuli, such as PTT, then release TAAs. The released TSAs/TAAs, together with the immune adjuvant (R837/Liposome), drive the maturation of DCs. The CCNPs also used the tumor-specific enzyme metallomatrix protease 2 (MMP2) to dramatically extend the half-life of the peptides, e.g. PD-L1 inhibitory peptides, or combined with PD-1/PD-L1 inhibitors to further enhance the anti-tumor effect. The above processes promote the infiltration of CTLs for treating distant metastasis. (C) Combination therapy based on ICIs: A combination therapeutic strategy that includes PDT, PTT and SDT, particularly robust anti-cancer agents loaded on CCNPs, results in enhanced anti-tumor effect of PD-1/PD-L1 inhibitors. (D) Cancer vaccines: As tumor vaccines, CCNPs can directly release TSAs/TAAs, release cytokines to promote the transformation of functional immune cells or are added special molecules on the cancer cell membrane to increase the antigen presentation to DCs, thus further enhancing the anti-tumor immune effect. Abbreviations: CCNPs = cancer cell membrane-wrapped nanoparticles, TIME = tumor immune microenvironment, TAAs = tumor-associated antigens, TSAs = tumor-specific antigens, PTT = photothermal therapy, PDT = photodynamic therapy, MMP2 = metallomatrix protease 2, PD-L1/PD-1 = programmed death-ligand 1/programmed death-1, CTLs = cytotoxic T lymphocytes, DCs = dendritic cells, ICIs = immune checkpoint inhibitors, NPs = nanoparticles, SDT = sonodynamic therapy, PDT = photodynamic therapy, Th1 cell = T helper 1 cell, CBP-12 = 12-mer Clec9a binding peptide.