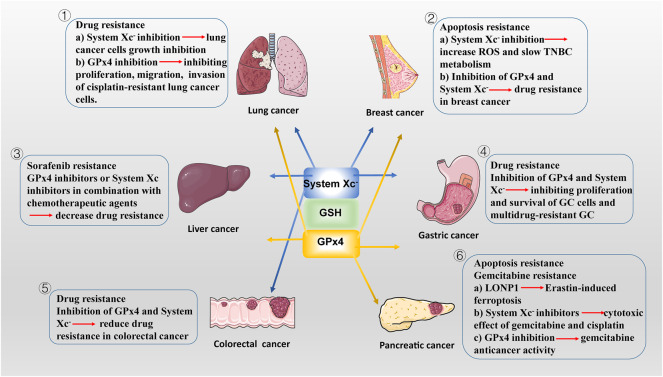
FIGURE 2.
The potencial roles of System Xc-/GSH/GPx4 in drug-resistant solid tumor. ① SLC7A11, highly expressed in NSCLC, is a potential target for ferroptosis. SLC7A11 downregulation lead to ferroptosis of lung cancer cells and inhibit their growth. In addition to SLC7A11, lung cancer cell also exhibits high GPx4 expression. GPX4 inhibitor limits proliferation, migration, and invasion of cisplatin-resistant lung cancer cells. ② Drug-resistant breast cancer cells are dependent on GPX4 and SLC7A11. SLC7A11 is upregulated in one-third of TNBC cells in vivo, and inhibiting System Xc-activity increases intracellular ROS levels and slows TNBC metabolism. Inhibition of GPX4 and/or System Xc-may be a potential measure to overcome drug resistance in breast cancer. ③ The main regulatory mediators mediating the ferroptotic response in HCC cells have been identified as System Xc-and GPX4. blocking System Xc -/GSH/GPX4 axis in combination with chemotherapeutic agents (e.g., sorafenib) provides new ideas for treatment of drug-resistant HCC. ④ GPx4 is lowly expressed in GC cells, making them more susceptible to ferroptosis than normal intestinal cells. Reducing the expression of GPX4 and System Xc-inhibiting the proliferation of GC cells and multidrug-resistant GC. ⑤ In CSCs, SLC7A11 is extremely expressed, with high GSH levels and low ROS levels, leading to their extreme vulnerability to ferroptosis. Similar to GC cells, targeting the System Xc-/GSH/GPX4 axis is an effective way to inhibit the growth of drug-resistant colorectal cancer. ⑥ LONP1 inhibits Nrf2-mediated GPX4 gene expression, thereby promoting Erastin-induced ferroptosis in human PDAC cells. The use of System Xc-inhibitors enhanced the cytotoxic effect of gemcitabine and cisplatin on PDAC cell lines. Gemcitabine resistance was associated with GPx4 upregulation in PDAC cells. Inhibition of GPX4 activity or induction of GPX4 degradation can restore or enhance the anticancer activity of gemcitabine in vitro or in xenogeneic PDAC models.