Figure 3.
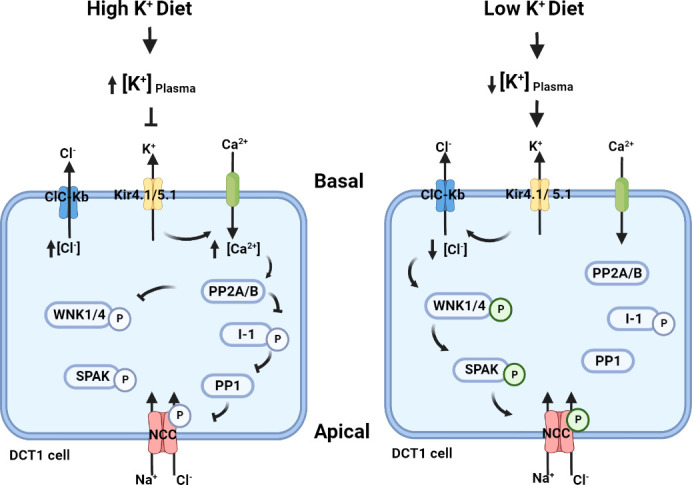
The putative “renal-K+ switch” mechanism. In the basolateral membrane, K+ channels Kir4.1/5.1 and a Cl- channel ClC-Kb can indirectly regulate NCC activity by modifying intracellular [Cl-] and hence the autophosphorylation of WNKs (145). High dietary K+ intake increases plasma [K+], resulting in reduced K+ extrusion by Kir4.1 and plasma membrane depolarisation of the early DCT (DCT1). This limits Cl- removal by ClC-Kb and hence intracellular Cl- mediated inhibition of the WNK-SPAK-NCC pathway remains (146–148). In addition, the effects on Kir4.1 may facilitate extracellular Ca2+ influx across the membrane via an unknown voltage-gated Ca2+ channel. This increased intracellular Ca2+ is proposed to activate protein phosphatase 2 (PP2A/B) to inhibit WNK (149, 150), and potentially PP2A/B may modulate the protein phosphatase 1 inhibitor (I-1) protein phosphatase 1 (PP1) pathway leading to NCC dephosphorylation (151–153). In contrast, a low K+ diet reduces the plasma K+ concentration. Low extracellular K+ results in cellular K+ extrusion by Kir4.1 leading to membrane hyperpolarization and release of Cl- from the cell through ClC-Kb. The subsequent reduction in intracellular Cl- relieves the inhibition of WNK4 autophosphorylation and allows the WNK-SPAK pathway to phosphorylate and activate NCC, leading to more NaCl reabsorption.
