Table 1. SAR of Trisubstituted 4,5-Dihydropyrazoles 1–44.
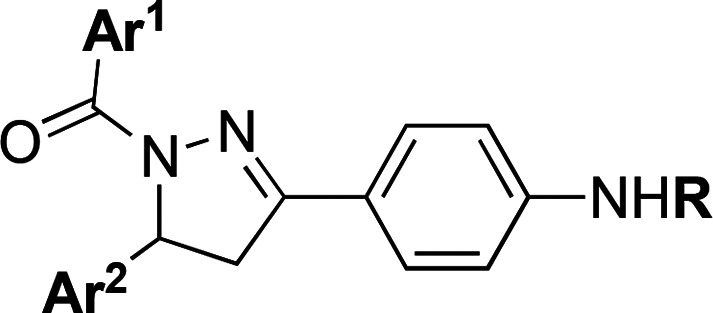
| compda | Ar1 | Ar2 | R | IC50 (μM)b |
|---|---|---|---|---|
| 1 | 4-MePh | 2-furyl | SO2Me | 15.9 ± 0.8 |
| 2 | 4-FPh | 2-furyl | SO2Me | 12.66 ± 0.22 |
| 3 | 4-ClPh | 2-furyl | SO2Me | 2.72 ± 0.11 |
| 4 | 4-BrPh | 2-furyl | SO2Me | 2.47 ± 0.36 |
| 5 | 4-CF3Ph | 2-furyl | SO2Me | 2.88 ± 0.06 |
| 6 | 4-NO2Ph | 2-furyl | SO2Me | 1.97 ± 0.12 |
| 7 | 4-CNPh | 2-furyl | SO2Me | 7.44 ± 0.21 |
| 8 | 4-MeOPh | 2-furyl | SO2Me | 10.11 ± 0.30 |
| 9 | Ph | 2-furyl | SO2Me | inactive |
| 10 | 3-MePh | 2-furyl | SO2Me | inactive |
| 11 | 3-FPh | 2-furyl | SO2Me | inactive |
| 12 | 2-furyl | 2-furyl | SO2Me | inactive |
| 13 | 4-MePh | 2-furyl | SO2CF3 | inactive |
| 14 | 4-MePh | 2-furyl | C(O)Me | inactive |
| 15 | 4-MePh | 2-furyl | H | inactive |
| 16 | 4-MePh | 2-furyl | CO2tBu | 3.29 ± 0.16 |
| 17 | 4-BrPh | 2-furyl | CO2tBu | 1.26 ± 0.03 |
| 18 | 4-BrPh | 3-furyl | CO2tBu | 3.82 ± 0.09 |
| 19 | 4-BrPh | thiazol-2-yl | CO2tBu | 1.61 ± 0.01 |
| 20 | 4-BrPh | thiazol-5-yl | CO2tBu | 4.36 ± 0.08 |
| 21 | 4-BrPh | 2-pyridinyl | CO2tBu | 0.65 ± 0.04 |
| 22 | 4-BrPh | 3-pyridinyl | CO2tBu | 1.63 ± 0.11 |
| 23 | 4-BrPh | 4-pyridinyl | CO2tBu | 2.78 ± 0.13 |
| 24 | 4-BrPh | Ph | CO2tBu | 0.262 ± 0.039 |
| 25 | 4-NO2Ph | Ph | CO2tBu | 0.439 ± 0.016 |
| 26 | 4-MeSO2Ph | Ph | CO2tBu | inactive |
| 27 | 4-MeC(O)Ph | Ph | CO2tBu | inactive |
| 28 | 4-tBuPh | Ph | CO2tBu | inactive |
| 29 | 4-CF3C(O)Ph | Ph | CO2tBu | inactive |
| 30 | 2-pyridinyl | Ph | CO2tBu | inactive |
| 31 | 3-pyridinyl | Ph | CO2tBu | 14.5 ± 3.7 |
| 32 | 4-pyridinyl | Ph | CO2tBu | inactive |
| 33 | 4-BrPh | Ph | CO2Me | 2.10 ± 0.07 |
| 34 | 4-BrPh | Ph | CO2Et | 0.0328 ± 0.0006 |
| 35 | 4-BrPh | Ph | CO2iPr | 0.0044 ± 0.0002 |
| 36 | 4-BrPh | Ph | CO2cPr | 0.0441 ± 0.0028 |
| 37 | 4-BrPh | Ph | CO2iBu | 0.164 ± 0.033 |
| 38 | 4-BrPh | Ph | CO2allyl | 0.0318 ± 0.0028 |
| 39 | 4-BrPh | Ph | CO2(CH2)2C2H | 0.065 ± 0.022 |
| 40 | 4-BrPh | Ph | C(O)NHMe | 5.53 ± 0.12 |
| 41 | 4-BrPh | Ph | C(O)NMe2 | 2.41 ± 0.03 |
| 42 | 4-BrPh | Ph | C(O)pyrrolidine | inactive |
| 43 | 4-BrPh | Ph | C(O)NHiPr | 0.350 ± 0.009 |
| 44 | 4-BrPh | Ph | C(O)NHtBu | 9.22 ± 0.51 |
All compounds are racemates.
IC50 values represent the half maximal (50%) inhibitory concentration for the Bcl-2–Beclin 1 BH3 interaction as determined in the AlphaLISA assay. Error represents SD (n = 3). All compounds were inactive (at 10 μM) against the Bcl-2–Bax BH3 interaction except compounds 34 (IC50 = 6.62 ± 0.27 μM), 35 (IC50 = 0.88 μM ± 0.04 μM), 36 (IC50 = 15.1 ± 0.04 μM), and 38 (IC50 = 8.83 ± 0.39 μM).
