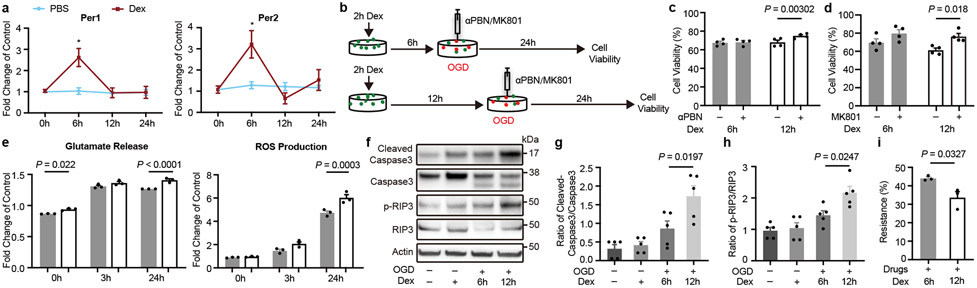
Figure 3. Effects of circadian cycles on response to oxygen-glucose deprivation and neuroprotection.
a. Primary mouse cortical neurons were treated for 2 hrs with dexamethasone (Dex). Per1/2 levels at 6 and 12 hrs matched in vivo circadian cycles for rats and mice at ZT15-21 (upregulated Per1/2) and ZT3-9 (downregulated Per1/2). *P=0.0003 (Per1), *P=0.0026 (Per2), n=3 independent experiments in triplicate. b. Oxygen-glucose deprivation (OGD) with αPBN or MK801 in neurons after induction of circadian-like cycles in vitro. c. αPBN was neuroprotective when Per1/2 were downregulated (n=4 independent experiments in triplicate). d. MK801 was neuroprotective when Per1/2 were downregulated (n=4 independent experiments in triplicate). e. After 3 hrs OGD, glutamate release and ROS production were lower during times with upregulated Per1/2 (n=3 independent experiments in triplicate). f. Representative western blots of cleaved caspase-3, caspase-3, phosphorylated RIP3 kinase and RIP3 kinase at 24 hrs after OGD (gel source data in Supplementary Information; n=5 independent experiments for densitometry). g. Quantitation of cleaved caspase-3/caspase-3. h. Quantitation of phosphorylated RIP3 kinase/RIP3 kinase. i. “Resistance to neuroprotection” after combination treatment with MK801 (10 μM), NBQX (50 μM), αPBN (1μM), zVAD-fmk (50 μM) and Necrostatin-1 (100 μM) (n=3 independent experiments in triplicate). All data in Figure 3 are mean ± SEM; two-way ANOVA with Bonferroni adjustment (a, c, d, e) or one-way ANOVA with post-hoc Tukey adjustment (g, h) or unpaired 2-tailed t-test (i).