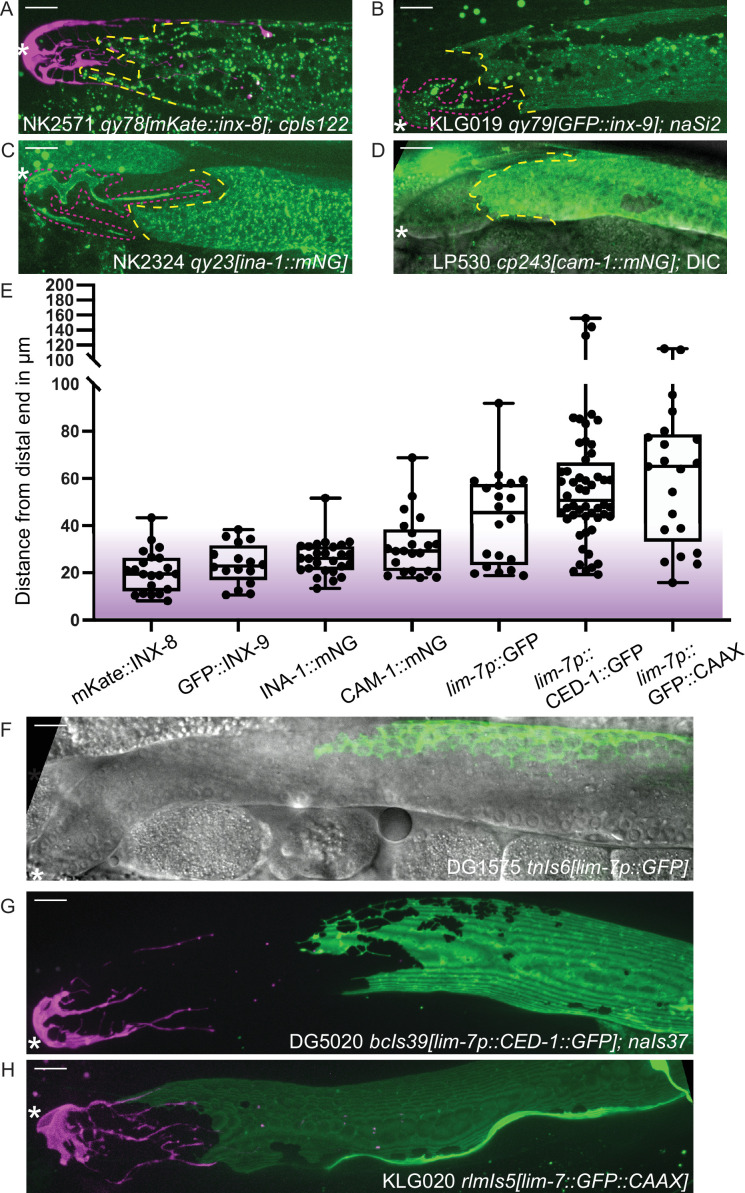
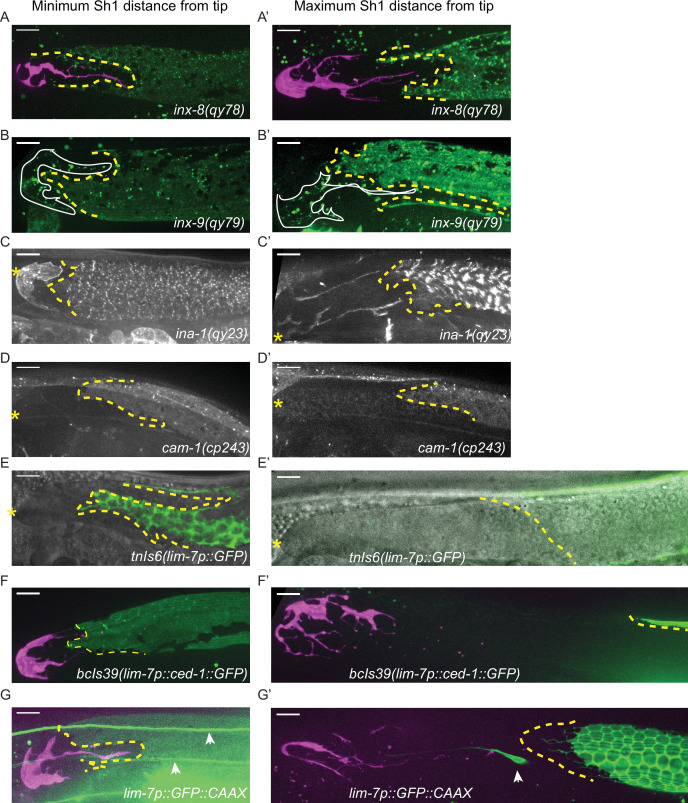
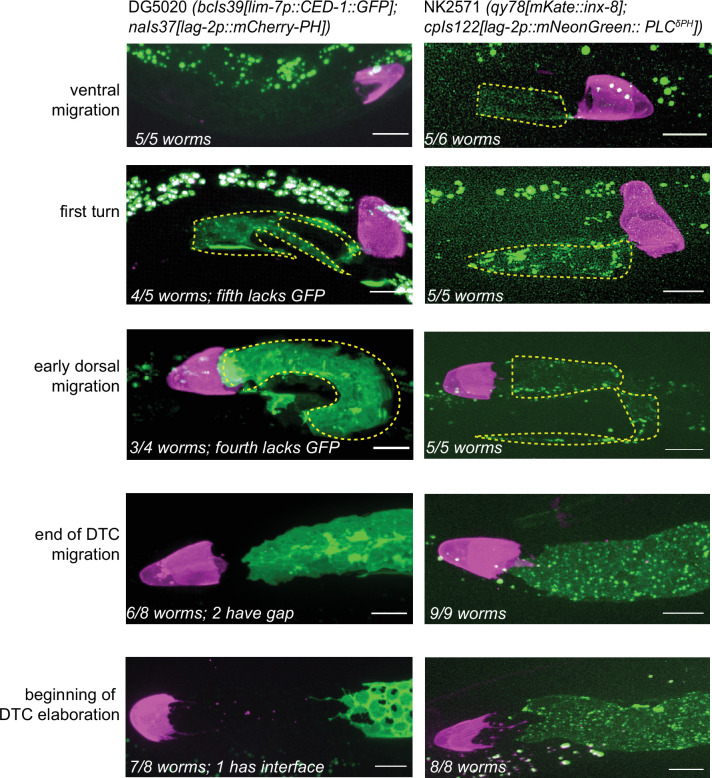
Figure 2. Sheath-expressed fluorescent proteins show consistency among endogenously tagged membrane proteins and greater variability in overexpressed transgenes.
(A) NK2571 qy78[mKate::inx-8]; cpIs122[lag-2p::mNeonGreen:: PLCδPH] N=21. (B) KLG019 qy79[GFP::inx9];naSi2 (channel not shown) N=16. (C) NK 2324 qy23[ina-1::mNG] N=26. (D) LP530 cp243[cam-1::mNG] N=21. (E) Box plots of Sh1 positions for all strains listed above and below, with fluorescent protein listed on the graph, including transgenes. (F) DG1575 tnIs6[lim-7p::GFP] N=20. (G) Strain DG5020 bcIs39[lim-7p::CED-1::GFP]; naIs37[lag-2p::mCherry-PH] N=52 (note that mean and range agree with those reported in Tolkin et al., 2022). (H) KLG020 rlmIs5[lim-7p::GFP::CAAX];cpIs122 N=21. Purple gradient marks approximate extent of stem cell zone (Lee et al., 2019; Shin et al., 2017). See Figure 2—figure supplement 1 for images of minimum and maximum observed distances for all markers. Figure 2—figure supplement 2 shows comparisons across development of NK2571 and DG5020. All scale bars 10 µm.