PURPOSE
Hematopoietic stem-cell transplantation–associated thrombotic microangiopathy (HSCT-TMA) is a serious complication with significant mortality and no approved therapy. HSCT-TMA results from endothelial injury, which activates the lectin pathway of complement. Narsoplimab (OMS721), an inhibitor of mannan-binding lectin-associated serine protease-2 (MASP-2), was evaluated for safety and efficacy in adults with HSCT-TMA.
METHODS
In this single-arm open-label pivotal trial (NCT02222545), patients received intravenous narsoplimab once weekly for 4-8 weeks. The primary end point (response rate) required clinical improvement in two categories: (1) laboratory TMA markers (both platelet count and lactate dehydrogenase) and (2) organ function or freedom from transfusion. Patients receiving at least one dose (full analysis set [FAS]; N = 28) were analyzed.
RESULTS
The response rate was 61% in the FAS population. Similar responses were observed across all patient subgroups defined by baseline features, HSCT characteristics, and HSCT complications. Improvement in organ function occurred in 74% of patients in the FAS population. One-hundred-day survival after HSCT-TMA diagnosis was 68% and 94% in FAS population and responders, respectively, whereas median overall survival was 274 days in the FAS population. Narsoplimab was well tolerated, and adverse events were typical of this population, with no apparent safety signal of concern.
CONCLUSION
In this study, narsoplimab treatment was safe, significantly improved laboratory TMA markers, and resulted in clinical response and favorable overall survival.
INTRODUCTION
Hematopoietic stem-cell transplantation–associated thrombotic microangiopathy (HSCT-TMA), also known as transplant-associated TMA, is a potentially fatal complication of HSCT that occurs when endothelial injury and microthrombus formation lead to thrombocytopenia, microangiopathic hemolytic anemia, and organ damage.1 It may coexist with other transplant complications such as graft-versus-host disease (GVHD), in which endothelial cells suffer aggravated injury.2 HSCT-TMA has a high rate of morbidity and mortality,1 and patients developing TMA post-transplantation show an increase in nonrelapse mortality.3 HSCT-TMA is under-recognized since anemia and thrombocytopenia are common after HSCT, which may contribute to a variable reported incidence up to 39% in patients undergoing allogeneic HSCT.4,5 Although under-recognized, HSCT-TMA can be accurately diagnosed by the unique history and combination of thrombocytopenia, greater-than-expected transfusion needs, elevated lactate dehydrogenase (LDH), schistocytes, and end-organ injury (eg, proteinuria, hypertension, elevated creatinine, or neurologic abnormalities). Other conditions can cause a subset of these findings, but current diagnostic criteria require a constellation of findings. The lack of approved therapy for HSCT-TMA is a significant unmet medical need.
CONTEXT
Key Objective
The lack of approved therapy for hematopoietic stem-cell transplantation–associated thrombotic microangiopathy (HSCT-TMA) represents a significant unmet need. Targeting the lectin pathway of complement is a novel approach for treatment of HSCT-TMA. Narsoplimab is a fully human monoclonal antibody that binds to and inhibits mannan-binding lectin-associated serine protease-2, the effector enzyme of the lectin pathway and an activator of the coagulation cascade. In this pivotal clinical trial, narsoplimab safety and efficacy were evaluated in patients with HSCT-TMA.
Knowledge Generated
Patients treated with at least one dose of narsoplimab had a response rate of 61%, as determined by improvement in both laboratory markers and clinical status. Sixty-eight percent of patients achieved 100-day survival after HSCT-TMA diagnosis.
Relevance
In this prospective study, improvement in all response criteria following narsoplimab treatment indicates clinically relevant resolution of HSCT-TMA pathophysiology. Data across patient subgroups suggest broad treatment potential of narsoplimab for HSCT-TMA.
HSCT causes substantial endothelial injury because of conditioning regimens, immunosuppressive treatment, infection, and alloreactivity.6,7 Syndromes arising from endothelial injury—collectively termed endothelial injury syndromes—can result in end-organ damage and failure.7 Common endothelial injury syndromes include veno-occlusive disease/sinusoidal obstruction syndrome, GVHD, and TMA.7,8
The complement system is activated by three distinct pathways: the classical, alternative, and lectin pathways. Inhibition of the lectin pathway is a potential therapeutic strategy for HSCT-TMA. Endothelial injury directly activates the lectin pathway of complement,9 which subsequently activates the terminal lytic pathway gated by C5. In patients with HSCT-TMA, the lectin pathway is activated.10 Mannan-binding lectin-associated serine protease-2 (MASP-2) is the effector enzyme of the lectin pathway and an activator of the coagulation cascade.11-13 MASP-2 levels are elevated following HSCT and in patients with HSCT-TMA.14 Inhibition of MASP-2 may provide therapeutic benefit for HSCT-TMA and potentially other endothelial injury syndromes.
Narsoplimab (OMS721) is a fully human immunoglobulin G4 monoclonal antibody that binds to and inhibits MASP-2. Narsoplimab, via MASP-2 inhibition, blocks lectin pathway activation while leaving the adaptive immune (ie, the classical pathway) response intact and fully functional.15 Herein, we report safety and efficacy of narsoplimab for the treatment of HSCT-TMA in the first formally established prospective clinical trial of patients at high risk for poor outcomes.
METHODS
Study Design
This was a phase II, single-arm, three-stage, ascending-dose-escalation study (NCT02222545) originally enrolling patients with any one of three forms of TMA: atypical hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, or HSCT-TMA. This study was initially designed as a proof-of-concept trial similar to phase I/phase II oncology studies. Following discussion with US Food and Drug Administration (FDA) and FDA's granting of Breakthrough Therapy Designation for narsoplimab in HSCT-TMA, the study was converted to a pivotal trial for this indication. The study as it relates to HSCT-TMA is the focus of this report.
The study was conducted in three stages (Data Supplement, online only). Stage I included patients with atypical hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, and HSCT-TMA, and had three ascending-dose cohorts to set the dose and allow assessment of safety. Patients received four once-weekly doses of narsoplimab; all HSCT-TMA patients in stage I received the high dose. Stage II was a cohort-expansion stage, providing four once-weekly doses of narsoplimab for HSCT-TMA patients, and stage III was an optional 4-week extension of dosing for patients who completed stage II, providing up to eight scheduled once-weekly doses of narsoplimab.
Narsoplimab was administered intravenously at a dose of 4 mg/kg once weekly, on the basis of evidence that this dosage provided > 80% inhibition of the lectin pathway throughout the dosing interval and was well tolerated.16 The large majority of study patients received weight-based dosing at 4 mg/kg but late in the study, the dose was changed to a fixed dose of 370 mg. The dose duration of 4-8 weeks was chosen on the basis of the acute nature of HSCT-TMA.
The core study period was defined as the study screening visit to last scheduled follow-up visit. The safety evaluation period was defined as duration from informed consent to 37 days after last narsoplimab dose.
This study was conducted in accordance with the principles of Good Clinical Practice, the Declaration of Helsinki, and local laws and regulations. The study was reviewed and approved by ethics committees and institutional review boards. All participants provided written informed consent.
Patients
Persons age ≥ 18 years at screening with a diagnosis of persistent HSCT-TMA were enrolled. Derived from criteria according to Cho et al,17 persistent HSCT-TMA was defined as meeting all of the following conditions for a period of ≥ 2 weeks after modification or discontinuation of calcineurin inhibitor (CNI), or ≥ 30 days after transplantation: (1) platelet count < 150 × 109 per liter; (2) evidence of microangiopathic hemolysis (presence of schistocytes, serum LDH above the upper limit of normal, or haptoglobin below the lower limit of normal); and (3) kidney dysfunction (doubling of serum creatinine from pretransplant). Key exclusion criteria were eculizumab therapy within 3 months before screening or concurrently with narsoplimab; Shiga toxin–producing Escherichia coli–associated hemolytic uremic syndrome; or a positive direct Coombs test. Full inclusion and exclusion criteria are provided in the Data Supplement.
End Points
The coprimary objectives for this study were to assess safety and evaluate response to narsoplimab in patients with HSCT-TMA. Safety and tolerability were assessed by adverse events, vital signs, electrocardiograms, and clinical laboratory tests.
The primary efficacy end point was response to narsoplimab, defined by coincident improvement in laboratory TMA markers and any one of several potential clinical benefits at any time after the first dose of narsoplimab (Fig 1). Specific thresholds were set regarding improvement in laboratory TMA markers. Clinical benefits of specific organ functions and transfusion burden were evaluated as possible, considering that patients had different patterns of organ dysfunction. This composite primary end point was developed in collaboration with and agreed upon by FDA.
FIG 1.
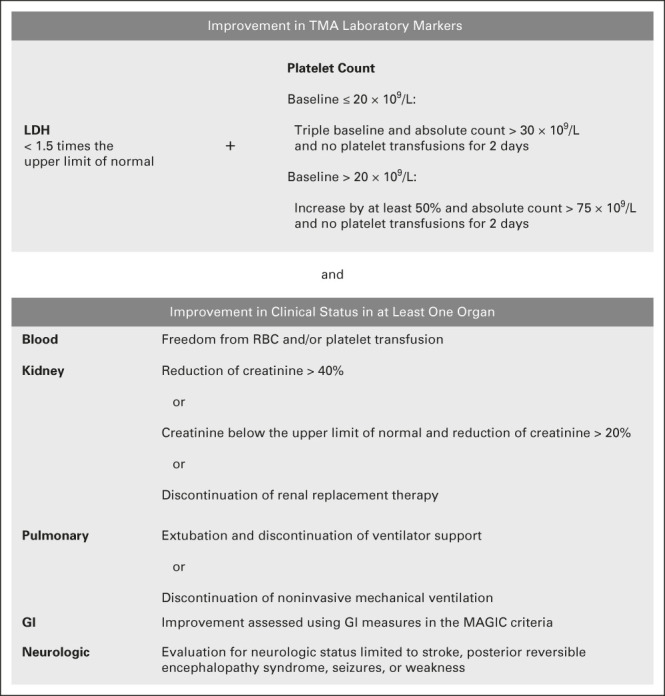
Definition of primary efficacy end point. Freedom from transfusion represented an absence of any combination of RBC transfusions and/or platelet transfusions for at least 4 weeks. Transfusion of any type was not an inclusion criterion; however, freedom from transfusion was included in the end point because it is a clinical benefit. GVHD, graft versus host disease; LDH, lactate dehydrogenase; MAGIC, Mount Sinai Acute GVHD International Consortium; TMA, thrombotic microangiopathy.
Key secondary end points were survival (100-day and overall) from the date of diagnosis of HSCT-TMA, change from baseline in laboratory markers (platelet count, LDH, haptoglobin, hemoglobin, and creatinine), and time to hematologic response (defined as the time from first narsoplimab dose to the first instance of achieving both platelet count and LDH response criteria).
Statistical Analysis
It was expected that approximately 28 patients with HSCT-TMA would be included in the efficacy analysis. The width of exact 95% CI for the response rate on the basis of 28 patients ranged from 12 to 39. If the observed response rate was 50%, the exact 95% CI would be 31 to 69. The sample size of three patients per dose escalation cohort in stage I was considered sufficient, in combination with data from phase I, to allow for dose selection. The sample size for stages II and III was determined empirically and agreed with FDA.
The full analysis set (FAS) population included patients with HSCT-TMA who received ≥ 1 dose of narsoplimab. The safety analysis set population included all patients who received any amount of narsoplimab.
Only patients with HSCT-TMA were included in efficacy and safety analyses. Summary statistics for continuous variables included number of patients, mean, median, standard deviation, minimum, maximum, and 95% CI. For categorical variables, number of patients, percentages, and 95% CI were provided.
The primary efficacy end point (response rate) was estimated with exact 95% CI for the FAS population. Time-weighted average analysis and repeated-measures models were used to estimate the mean change from baseline over time for continuous secondary efficacy end points. One-hundred-day survival status was determined as a binary variable and was estimated with exact 95% CI. Overall survival was analyzed using Kaplan-Meier methods. Nonresponder imputation was planned to handle missing data for binary efficacy end points. No multiplicity adjustment was performed.
RESULTS
Patients
Twenty-eight patients with HSCT-TMA were enrolled between August 2015 and October 2019. The last patient completed the study in January 2020. All enrolled patients with HSCT-TMA received ≥ 1 dose of narsoplimab (FAS population). Patient disposition is shown in Figure 2 and represents patients who completed study drug administration as defined by the assigned treatment (ie, eight once-weekly doses in stage III). No patients had missing primary efficacy end point data.
FIG 2.
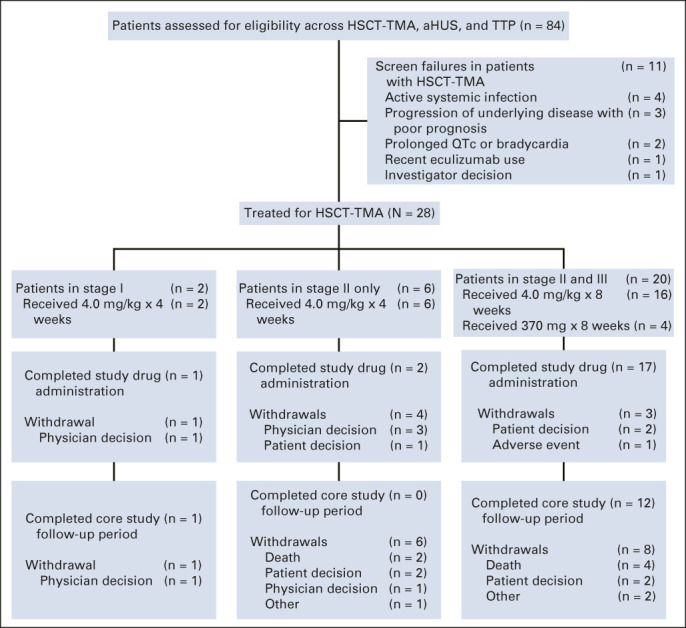
Flow diagram. Patient disposition during the core study period. Completion of study drug administration was defined by the assigned treatment (ie, eight once-weekly doses in stage III). aHUS, atypical hemolytic uremic syndrome; HSCT-TMA, hematopoietic stem-cell transplantation–associated thrombotic microangiopathy; QTc, corrected QT interval; TTP, thrombotic thrombocytopenic purpura.
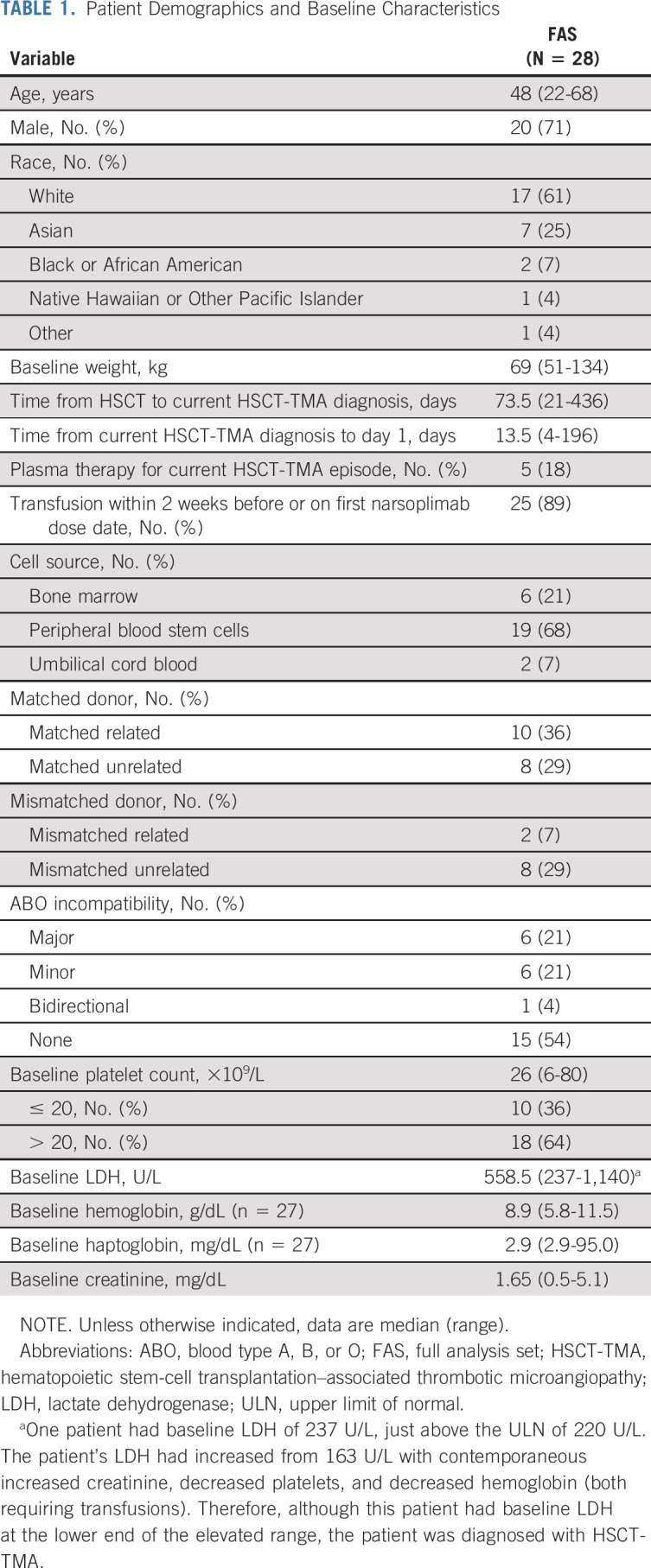
All patients were adults who had undergone allogeneic HSCT (Table 1). Median age was 48 years (range, 22-68 years) and 71% of patients were male. There were no significant differences in baseline weight or body mass index between patients who received the weight-based dose of 4 mg/kg (24 patients) and those who received a fixed dose of 370 mg (four patients). All but one of the patients had a hematologic malignancy. The large majority of patients had multiple risk factors for poor outcomes at baseline, including significant infections, kidney dysfunction, GVHD, neurologic dysfunction, multiple organ TMA involvement, or pulmonary dysfunction (Data Supplement).
TABLE 1.
Patient Demographics and Baseline Characteristics
Primary Efficacy End Point
All patients who received ≥ 1 dose of narsoplimab (N = 28) were evaluated for efficacy. Seventeen patients responded to narsoplimab treatment on the basis of improvements in both laboratory TMA markers and clinical benefit, corresponding to a response rate of 61% (95% CI, 41 to 79) in the FAS population (Table 2).
TABLE 2.
Primary Efficacy End Point: Response to Narsoplimaba
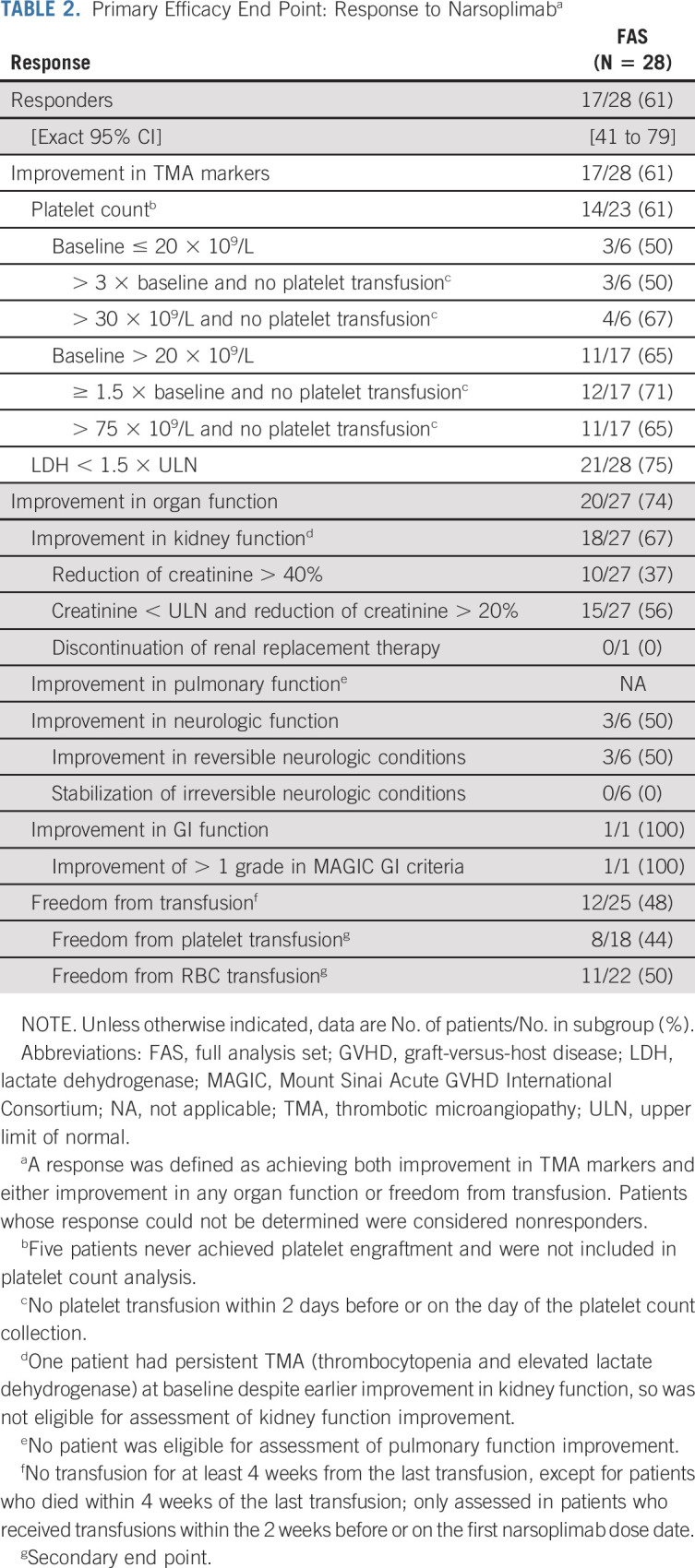
The FAS group's response rate was summarized by baseline demographics, clinicopathologic features, and HSCT complications post hoc to understand the effect of narsoplimab on subgroups (Data Supplement). The response rate for patients age < 65 years and those age 65 years or older was 58% and 75%, respectively. Response rates for patients with GVHD, significant infections, and multiple organ TMA involvement were 63%, 63%, and 64%, respectively. Response rates for patients with kidney, pulmonary, or GI dysfunctions were 57%, 40%, and 100%, respectively.
Response across components of the primary end point was also consistent. Improvement in laboratory TMA markers occurred in 17 patients in the FAS population (61%). Improvement in organ function occurred in 20 of 27 patients (74%) in the FAS population (Table 2). One patient had persistent TMA (thrombocytopenia and elevated LDH) at baseline despite earlier improvement in kidney function, so was not eligible for assessment of kidney function improvement. Freedom of transfusion was achieved by 12 of 25 patients (48%) in the FAS population; three patients were not receiving transfusions at baseline, meaning they were not eligible for freedom from transfusion assessment.
Secondary End Points
One-hundred-day survival after HSCT-TMA diagnosis was 68% (95% CI, 48 to 84) in the FAS population and 94% (95% CI, 71 to 100) for responders (Fig 3A). Median overall survival was 274 days (95% CI, 103 to not estimable) in the FAS (Fig 3B).
FIG 3.
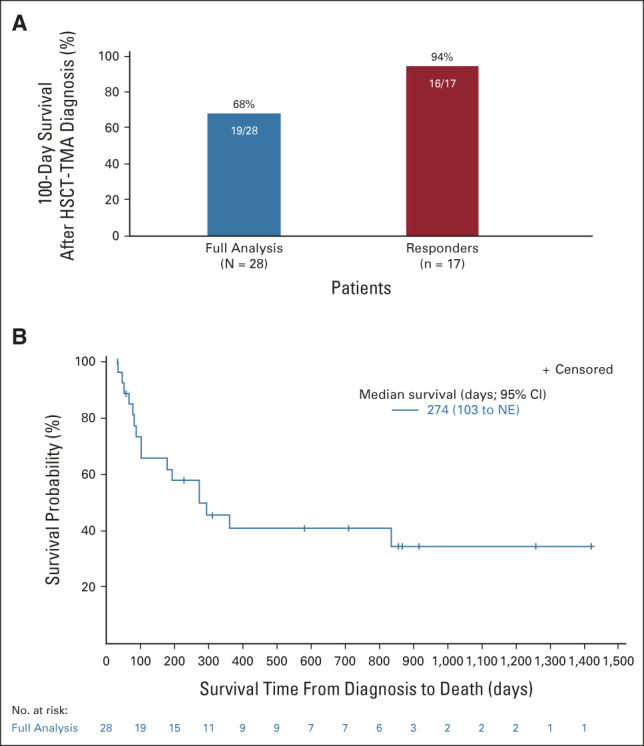
Survival with narsoplimab treatment. (A) 100-day survival after HSCT-TMA diagnosis. (B) Kaplan-Meier plot of overall survival after HSCT-TMA diagnosis. HSCT-TMA, hematopoietic stem-cell transplantation–associated thrombotic microangiopathy; NE, not estimable.
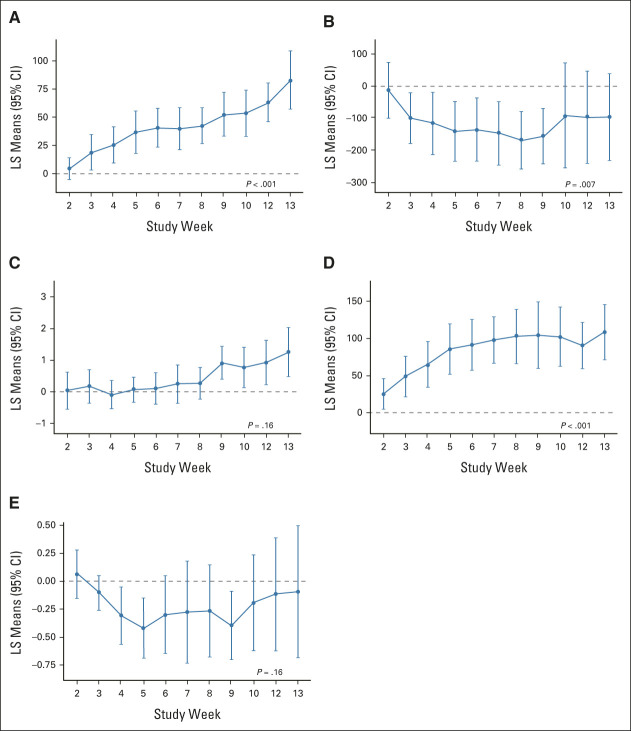
Median time to reach hematologic response was 36 days (range, 7-228 days) from first narsoplimab dose. Fifteen of 17 responders demonstrated a response during narsoplimab treatment or within 14 days of stopping treatment. The remaining two patients had coexisting conditions suppressing platelet counts; response was apparent following resolution of these conditions. The mean time-weighted average change from baseline in laboratory markers (FAS population) was calculated for platelet count (29.5 × 109/L [95% CI, 13.5 to 45.6; P < .001]), LDH (–111.9 U/L [–190.7 to –33.1; P = .007]), hemoglobin (0.38 g/dL [–0.17 to 0.92; P = .164]), haptoglobin (67.7 mg/dL [40.2 to 95.2; P < .001]), and creatinine (–0.18 mg/dL [–0.43 to 0.07; P = .156]). The least squares mean change from baseline over time for these markers is shown in Figure 4.
FIG 4.
Change in laboratory markers. LS mean change from baseline over time for FAS: (A) platelet count (109 per L), (B) LDH (U/L), (C) hemoglobin (g/dL), (D) haptoglobin (mg/dL), and (E) creatinine (mg/dL). No data were imputed; all available data were included. P-values from time-weighted average change from baseline using one-sample t-test. FAS, full analysis set; LDH, lactate dehydrogenase; LS, least squares.
Safety
In this study, the most commonly reported treatment-emergent adverse events were pyrexia, diarrhea, vomiting, nausea, neutropenia, fatigue, and hypokalemia (Table 3). Treatment-emergent adverse events, adverse events of grade 3 or higher, and treatment-related adverse events are provided in the Data Supplement.
TABLE 3.
Adverse Eventsa
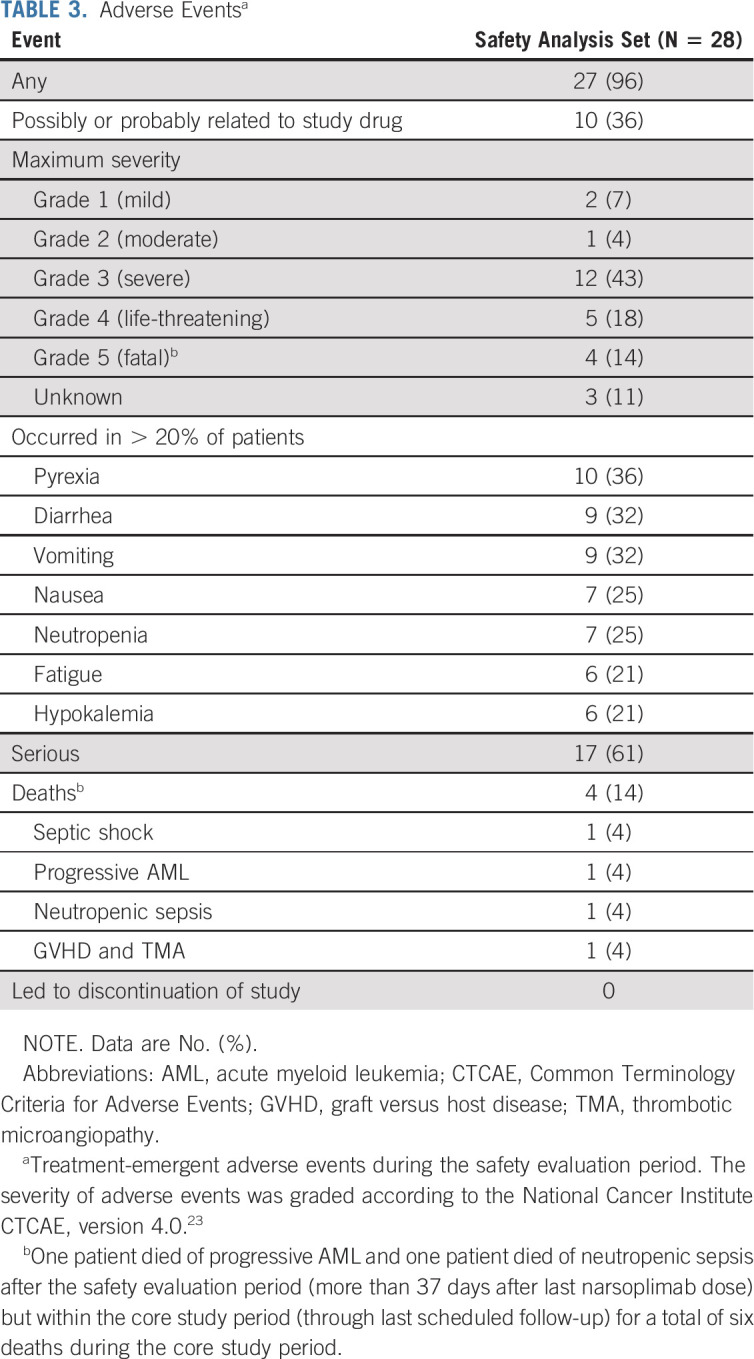
The most commonly reported type of adverse event was infection in 71% of patients, including grade 2 or higher infection in 61% of patients. The most common grade 2 or higher infections were cytomegalovirus, lower respiratory tract infection, pneumonia, neutropenic sepsis, Klebsiella pneumoniae infection, septic shock, and staphylococcal bacteremia. All reported grade 2 or higher infections were reviewed for severity and coexisting risk factors (ie, corticosteroid use, other immunosuppressive use, neutropenia, intravenous catheterization, and kidney failure) present at the start of infection. Twelve grade 2 (moderate) infections occurred in patients with at least two risk factors, nine occurred in patients with one risk factor, and one occurred in a patient with no risk factors. Fourteen grade 3 (severe) or higher infections occurred in patients with at least two risk factors, six occurred in patients with one risk factor (including three in patients whose only risk factor was low-dose steroids), and one occurred in a patient with no risk factors.
Treatment-emergent serious adverse events are listed in the Data Supplement. The highest percentage of serious adverse events by system organ class were infections (36% of patients); immune system disorders (18%); and respiratory, thoracic, and mediastinal disorders (18%). Six deaths occurred during the core study period: one patient died of septic shock (3 days after their last narsoplimab dose), two patients each died of progressive acute myeloid leukemia (6 and 40 days after their last dose) and of neutropenic sepsis (14 and 42 days after their last dose), and one patient died of GVHD and TMA (20 days after their last dose). On the basis of follow-up data collection, 10 deaths occurred after the study period: three patients died of disease progression; one patient each died of neutropenic sepsis, GVHD/infection, pneumonia, cardiopulmonary arrest; and three patients had an unknown cause of death.
Apart from the patients who died during the study period, no patient withdrew from the study for an adverse event.
DISCUSSION
In this pivotal clinical trial of adult patients with high-risk HSCT-TMA characterized by the presence of multiple risk factors (Data Supplement), narsoplimab treatment resulted in a 61% response rate in the FAS population, as defined by improvement in both laboratory TMA markers and clinical status (including freedom from transfusion). Improvement in TMA markers demonstrates the impact of narsoplimab treatment on underlying HSCT-TMA pathophysiology by decreasing platelet consumption and intravascular hemolysis. Additional improvements in organ function or freedom from transfusion support clinical benefit in these patients. Taken together, improvement in all response criteria following narsoplimab treatment indicates clinically relevant resolution of HSCT-TMA pathophysiology.
The primary end point was novel and, therefore, historical data were not available for comparison. After review of the severity of the patient population, experts estimated a probable primary end point response rate of approximately 15% and 100-day survival not exceeding 20%. Both the point estimates and lower bounds of the 95% CIs of both response rate and 100-day survival markedly exceeded expert estimates. The rationale for targeting MASP-2 inhibition for the treatment of HSCT-TMA is supported by the presence of elevated MASP-2 levels in patients with TMA.14
Notably, a consistent response was observed in all patient subgroups, indicating the effect of narsoplimab regardless of baseline characteristics. This finding suggests broad treatment potential for patients with HSCT-TMA.
Although some patients experienced haptoglobins in the normal range at baseline, this is not unexpected. Haptoglobin is an acute phase protein and can be elevated during early HSCT-TMA.18 Decreased haptoglobin has been reported to lag other diagnostic parameters such as LDH and renal dysfunction; normal haptoglobins have been observed more frequently in patients with severe disease.5 Therefore, normal baseline haptoglobins do not affect diagnosis, particularly when evaluated at the population level.
The high response rate to narsoplimab was associated with substantial survival in this high-risk patient population. One-hundred-day survival with narsoplimab treatment was 68% and median overall survival was 274 days in the FAS population. All except one of the responders achieved one-hundred-day survival (94%), demonstrating the durability and clinical relevance of the response. These results further support the hypothesis that narsoplimab treatment contributes to resolution of HSCT-TMA pathophysiology.
Narsoplimab was well tolerated, and no safety signals of concern were observed. Reported adverse events were consistent with typical adverse events seen in immunosuppressed post-transplantation patients. Given that this population was at high risk of severe and life-threatening infections, patients were closely monitored for infections during narsoplimab treatment. The most common adverse event was infection, although most patients experiencing severe infection had multiple risk factors for infection. Although the sample size was small, no evidence of increased infection risk because of narsoplimab was observed.
The main limitation of this trial was the single-arm study design. Because of the serious nature of the disease, it would be impractical to include a placebo arm in the trial. Although control arms are typically included in pivotal clinical trials, these are not always required in orphan diseases.19,20 Another limitation was the small sample size, related to the fact that HSCT-TMA is under-reported and this trial only included patients at high risk for poor outcomes. Finally, biomarkers were not assessed during this study.
Strengths of this trial include the novel yet rigorous primary efficacy end point. Laboratory TMA markers were objective and not subject to interpretation, whereas organ function and freedom from transfusion were evaluated by objective measures with little risk for bias. Furthermore, this trial did not exclude patients possessing risk factors for the development of HSCT-TMA, such as transplantation complications including GVHD, refractory underlying disease or relapse, recent controlled infections, or persistent TMA following CNI treatment modification. Inclusion of these patients allowed for evaluation of narsoplimab in an HSCT-TMA population at high risk for poor outcomes and highly reflective of real-world clinical practice. Studies have shown that CNI modification/withdrawal does not increase risk of HSCT-TMA development, nor does it affect resolution of HSCT-TMA or mortality.4,21 In keeping with the recent literature, multiple laboratory and clinical diagnostic criteria22 were adopted in our study to rule out the possibility that toxicity mediated by CNIs could be overinterpreted as true HSCT-TMA.
In summary, treatment with narsoplimab resulted in high response rates in patients with HSCT-TMA without apparent safety concerns. No differences were observed in safety and efficacy between the two dosing regimens. Similar responses were observed across all patient subgroups, and survival was substantial in this high-risk patient population.
ACKNOWLEDGMENT
The authors would like to acknowledge Mark Smith, MD, of Christchurch Hospital, New Zealand, for his role as a principal investigator in this study before his passing. Medical writing was provided by Charlotte A. Osborne, PhD, CMPP, of Omeros Corporation. Editorial assistance and figure support were provided by PharmaScribe, LLC. A complete list of members of the OMS721-TMA-001 Study Group is provided in Appendix 1 (online only).
APPENDIX 1. LIST OF PRINCIPAL INVESTIGATORS AND SUBINVESTIGATORS
Complete List of Principal Investigators for HSCT-TMA
Kathleen Claes (University Hospitals Leuven), Peter Ganly (Christchurch Hospital), Yeow Tee Goh (Singapore General Hospital), Samer K. Khaled (City of Hope), Yok Lam Kwong (Queen Mary Hospital), Nelson Leung (Mayo Clinic), Włodzimierz Mendrek (Maria Skłodowska-Curie National Research Institute of Oncology), Ara Metjian (Duke University Medical Center), Ryotaro Nakamura (City of Hope), Thomas L. Ortel (Duke University Medical Center), Alessandro Rambaldi (Hospital Papa Giovanni XXIII), Jameela Sathar (Ampang Hospital), and Mark Smith (Christchurch Hospital).
Complete List of Subinvestigators for HSCT-TMA
Duke University Medical Center: Gowthami Arepally and Nirmish Shah; City of Hope: Monzr M. Al Malki, Ibrahim T. Aldoss, Haris Ali, Ahmed Aribi, Ravi Bhatia, Lihua Elizabeth Budde, Ji-Lian Cai, Thai Minh Cao, Robert Wei-I Chen, Leonardo Torres Farol, Stephen Joel Forman, Alex Francisco Herrera, Myo Htut, Chatchada Karanes, Nicole A. Karras, Amrita Yeshoda Krishnan, Guido Marcucci, Matthew Genyeh Mei, Auayporn Nademanee, Nitya Atul Nathwani, Liana Nikolaenko, Margaret Ruth O'Donnell, Pablo Miguel Parker, Anna Beata Pawlowska, Leslie Lynn Popplewell, Vinod A. Pullarkat, Joseph Rosenthal, Michael Alan Rosenzweig, Firoozeh Sahebi, Amandeep Salhotra, Karamjeet Singh Sandhu, Tanya Siddiqi, Eileen Patricia Smith, David Samuel Snyder, George Somlo, Ricardo Tomas Spielberger, Anthony Selwyn Stein, and Jasmine M Zain; Mayo Clinic: Stephen M. Ansell, David Dingliv, Angela Dispenzieri, Fernando C. Fervenza, Morie A. Gertz, Ronald S. Go, Shahrukh K. Hashmi, Suzanne R. Hayman, William J. Hogan, C. Christopher Hook, Patrick B. Johnston, Martha Q. Lacy, Mark R. Litzow, Ivana N. Micallef, Mrinal S. Patnaik, and Luis F. Porrata; Maria Skłodowska-Curie National Research Institute of Oncology: Jacek Najda; University Hospitals Leuven: Bert Bammens, Daan Dierickx, Pieter Evenepoel, Dirk Kuypers, Bjorn Meijers, Maarten Naesens, and Ben Sprangers; Hospital Papa Giovanni XXIII: Alessandra Algarotti and Maria Caterina Mico; Christchurch Hospital: Andrew Butler, Stephen Gibbons, Amy Holmes, Wei-Hsun Hsu, Sean MacPherson, Emma-Jane McDonald, and Ruth Spearing; Queen Mary Hospital: Sau Yan Thomas Chan, Harinder Singh Gill, Yu Yan Hwang, and Joycelyn Pui Yin Sim; Ampang Hospital: Kim Wah Ho, Tze Shin Leong, Jay Suriar Rajasuriar, Loh Weng Khean, and Shahada Sobah binti Abdul Hamid; Singapore General Hospital: Chuen Wen Tan, Francesca Lim, Hartirathpal Kaur, Zhentang Lao, Jeffrey Quek, Lawrence Ng, Melinda Tan, Tertius Tuy, Colin Phipps Diong, Jason Choo Chon Jun, Mathini Jayaballa, and Mya Hae Tha.
Samer K. Khaled
Employment: Janssen Oncology
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Consulting or Advisory Role: Omeros
Speakers' Bureau: Alexion Pharmaceuticals
Research Funding: Daiichi Sankyo/Astra Zeneca, Alexion Pharmaceuticals, Genentech, Otsuka
Travel, Accommodations, Expenses: Alexion Pharmaceuticals
Kathleen Claes
Consulting or Advisory Role: Alexion Pharmaceuticals, Astellas Pharma, Sanofi
Yeow Tee Goh
Honoraria: AbbVie, MSD, Roche, Astellas Pharma, Johnson & Johnson/Janssen, NS Pharma
Consulting or Advisory Role: Novartis, AstraZeneca, Johnson & Johnson/Janssen, Antengene, Amgen, EUSA Pharma
Yok Lam Kwong
Honoraria: Amgen, Astellas Pharma, BeiGene, Bristol Myers Squibb/Celgene, Celgene, Janssen, Merck, Novartis, Roche, Takeda
Consulting or Advisory Role: Bristol Myers Squibb/Celgene, Celgene, Novartis, Roche
Speakers' Bureau: Novartis, Roche, Takeda
Research Funding: Merck, Novartis
Nelson Leung
Stock and Other Ownership Interests: Senseonics
Research Funding: Omeros
Włodzimierz Mendrek
Honoraria: Gilead Sciences
Research Funding: Janssen, Novartis
Travel, Accommodations, Expenses: Gilead Sciences
Ryotaro Nakamura
Consulting or Advisory Role: Viracor Eurofins, Magenta Therapeutics, Kadmon, Napajen Pharma, Omeros, Bluebird Bio
Research Funding: Helocyte (Inst), Miyarisan pharmaceutical (Inst)
Travel, Accommodations, Expenses: Kyowa Hakko Kirin, Alexion Pharmaceuticals
Jameela Sathar
Honoraria: Zuellig harma, Sysmex
Edmund Ng
Stock and Other Ownership Interests: Amgen, Cardinal Health
Consulting or Advisory Role: Blaze Bioscience, Omeros, Topcon
Travel, Accommodations, Expenses: Omeros
Narinder Nangia
Employment: Omeros
Stock and Other Ownership Interests: Omeros
Steve Whitaker
Employment: Omeros
Leadership: Omeros
Stock and Other Ownership Interests: Omeros
Patents, Royalties, Other Intellectual Property: Patents related to treatment of HSCT-TMA by narsoplimab
Travel, Accommodations, Expenses: OmerosCorporation
Alessandro Rambaldi
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript
Consulting or Advisory Role: Amgen, Omeros, Novartis, Astellas Pharma, Jazz Pharmaceuticals, Roche, AbbVie, Janssen, Pfizer, Incyte, Kite/Gilead
No other potential conflicts of interest were reported.
DISCLAIMER
The sponsor provided financial support for the study and participated in the study design; conducting the study; data collection, management, analysis, and interpretation; and preparation and review of the manuscript.
PRIOR PRESENTATION
Presented at 25th European Hematology Association Congress, virtual, June 11-21, 2020; 2021 Transplantation & Cellular Therapy Meetings of ASTCT & CIBMTR, virtual, February 8-12, 2021; 47th Annual Meeting of the European Society for Blood and Marrow Transplantation, virtual, March 14-17, 2021; 26th European Hematology Association Congress, virtual, June 9-17, 2021; and 28th International Complement Workshop, virtual, December 6-10, 2021.
SUPPORT
Supported by Omeros Corporation, Seattle, WA.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Yok Lam Kwong, Ryotaro Nakamura, Narinder Nangia, Steve Whitaker, Alessandro Rambaldi
Administrative support: Yeow Tee Goh
Provision of study materials or patients: Kathleen Claes, Yeow Tee Goh, Yok Lam Kwong, Ryotaro Nakamura, Alessandro Rambaldi
Collection and assembly of data: Samer K. Khaled, Yeow Tee Goh, Yok Lam Kwong, Nelson Leung, Włodzimierz Mendrek, Narinder Nangia, Steve Whitaker, Alessandro Rambaldi
Data analysis and interpretation: Samer K. Khaled, Kathleen Claes, Yeow Tee Goh, Yok Lam Kwong, Ryotaro Nakamura, Jameela Sathar, Edmund Ng, Narinder Nangia, Steve Whitaker, Alessandro Rambaldi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Narsoplimab (OMS721), a Mannan-Binding Lectin-Associated Serine Protease-2 Inhibitor, for the Treatment of Adult Hematopoietic Stem-Cell Transplantation–Associated Thrombotic Microangiopathy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Samer K. Khaled
Employment: Janssen Oncology
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Consulting or Advisory Role: Omeros
Speakers' Bureau: Alexion Pharmaceuticals
Research Funding: Daiichi Sankyo/Astra Zeneca, Alexion Pharmaceuticals, Genentech, Otsuka
Travel, Accommodations, Expenses: Alexion Pharmaceuticals
Kathleen Claes
Consulting or Advisory Role: Alexion Pharmaceuticals, Astellas Pharma, Sanofi
Yeow Tee Goh
Honoraria: AbbVie, MSD, Roche, Astellas Pharma, Johnson & Johnson/Janssen, NS Pharma
Consulting or Advisory Role: Novartis, AstraZeneca, Johnson & Johnson/Janssen, Antengene, Amgen, EUSA Pharma
Yok Lam Kwong
Honoraria: Amgen, Astellas Pharma, BeiGene, Bristol Myers Squibb/Celgene, Celgene, Janssen, Merck, Novartis, Roche, Takeda
Consulting or Advisory Role: Bristol Myers Squibb/Celgene, Celgene, Novartis, Roche
Speakers' Bureau: Novartis, Roche, Takeda
Research Funding: Merck, Novartis
Nelson Leung
Stock and Other Ownership Interests: Senseonics
Research Funding: Omeros
Włodzimierz Mendrek
Honoraria: Gilead Sciences
Research Funding: Janssen, Novartis
Travel, Accommodations, Expenses: Gilead Sciences
Ryotaro Nakamura
Consulting or Advisory Role: Viracor Eurofins, Magenta Therapeutics, Kadmon, Napajen Pharma, Omeros, Bluebird Bio
Research Funding: Helocyte (Inst), Miyarisan pharmaceutical (Inst)
Travel, Accommodations, Expenses: Kyowa Hakko Kirin, Alexion Pharmaceuticals
Jameela Sathar
Honoraria: Zuellig harma, Sysmex
Edmund Ng
Stock and Other Ownership Interests: Amgen, Cardinal Health
Consulting or Advisory Role: Blaze Bioscience, Omeros, Topcon
Travel, Accommodations, Expenses: Omeros
Narinder Nangia
Employment: Omeros
Stock and Other Ownership Interests: Omeros
Steve Whitaker
Employment: Omeros
Leadership: Omeros
Stock and Other Ownership Interests: Omeros
Patents, Royalties, Other Intellectual Property: Patents related to treatment of HSCT-TMA by narsoplimab
Travel, Accommodations, Expenses: OmerosCorporation
Alessandro Rambaldi
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript
Consulting or Advisory Role: Amgen, Omeros, Novartis, Astellas Pharma, Jazz Pharmaceuticals, Roche, AbbVie, Janssen, Pfizer, Incyte, Kite/Gilead
No other potential conflicts of interest were reported.
REFERENCES
- 1.Pagliuca S, Michonneau D, de Fontbrune FS, et al. : Allogeneic reactivity-mediated endothelial cell complications after HSCT: A plea for consensual definitions. Blood Adv 3:2424-2435, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dietrich S, Falk CS, Benner A, et al. : Endothelial vulnerability and endothelial damage are associated with risk of graft-versus-host disease and response to steroid treatment. Biol Blood Marrow Transplant 19:22-27, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Shayani S, Palmer J, Stiller T, et al. : Thrombotic microangiopathy associated with sirolimus level after allogeneic hematopoietic cell transplantation with tacrolimus/sirolimus-based graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant 19:298-304, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postalcioglu M, Kim HT, Obut F, et al. : Impact of thrombotic microangiopathy on renal outcomes and survival after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 24:2344-2353, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jodele S, Davies SM, Lane A, et al. : Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: A study in children and young adults. Blood 124:645-653, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Storb R: HSCT: Historical perspective, in Carreras E, Dufour C, Mohty M, et al. (eds): The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. Cham, CH, Springer, 2019 [PubMed] [Google Scholar]
- 7.Carreras E, Diaz-Ricart M: The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplant 46:1495-1502, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Hildebrandt GC, Chao N: Endothelial cell function and endothelial-related disorders following haematopoietic cell transplantation. Br J Haematol 190:508-519, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collard CD, Väkevä A, Morrissey MA, et al. : Complement activation after oxidative stress: Role of the lectin complement pathway. Am J Pathol 156:1549-1556, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galbusera M, Gastoldi S, Noris M, et al. : In acute HSCT/BMT-TMA the activation of the lectin pathway induces C5b-9 formation on endothelial cells and favors microvascular thrombosis. Bone Marrow Transplant 56:295-297, 2021 [Google Scholar]
- 11.Kozarcanin H, Lood C, Munthe-Fog L, et al. : The lectin complement pathway serine proteases (MASPs) represent a possible crossroad between the coagulation and complement systems in thromboinflammation. J Thromb Haemost 14:531-545, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Gulla KC, Gupta K, Krarup A, et al. : Activation of mannan-binding lectin-associated serine proteases leads to generation of a fibrin clot. Immunology 129:482-495, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krarup A, Wallis R, Presanis JS, et al. : Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS One 2:e623, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elhadad S, Chapin J, Copertino D, et al. : MASP2 levels are elevated in thrombotic microangiopathies: Association with microvascular endothelial cell injury and suppression by anti-MASP2 antibody narsoplimab. Clin Exp Immunol 203:96-104, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwaeble WJ, Lynch NJ, Clark JE, et al. : Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc Natl Acad Sci 108:7523-7528, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman J, Cummings J, Chuidian M, et al. : Development of pharmacodynamic assays to assess ex vivo MASP-2 inhibition and their use to characterize the pharmacodynamics of narsoplimab (OMS721) in humans and monkeys. Blood 136:26-27, 2020 [Google Scholar]
- 17.Cho B-S, Yahng S-A, Lee S-E, et al. : Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation 90:918-926, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Schuh MP, Bennett MR, Lane A, et al. : Haptoglobin degradation product as a novel serum biomarker for hematopoietic stem cell transplant-associated thrombotic microangiopathy. Pediatr Nephrol 34:865-871, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasinowski FJ, Panico EB, Valentine JE: Quantum of effectiveness evidence in FDA's approval of orphan drugs: Update, July 2010 to June 2014. Ther Innov Regul Sci 49:680-697, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Gobburu J, Pastoor D: Drugs against rare diseases: Are the regulatory standards higher? Clin Pharmacol Ther 100:322-323, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li A, Wu Q, Davis C, et al. : Transplant-associated thrombotic microangiopathy is a multifactorial disease unresponsive to immunosuppressant withdrawal. Biol Blood Marrow Transplant 25:570-576, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dandoy CE, Rotz S, Alonso PB, et al. : A pragmatic multi-institutional approach to understanding transplant-associated thrombotic microangiopathy after stem cell transplant. Blood Adv 5:1-11, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Department of Health and Human Services : Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Bethesda, MD, National Institutes of Health, National Cancer Institute, 2009 [Google Scholar]