FIG 2.
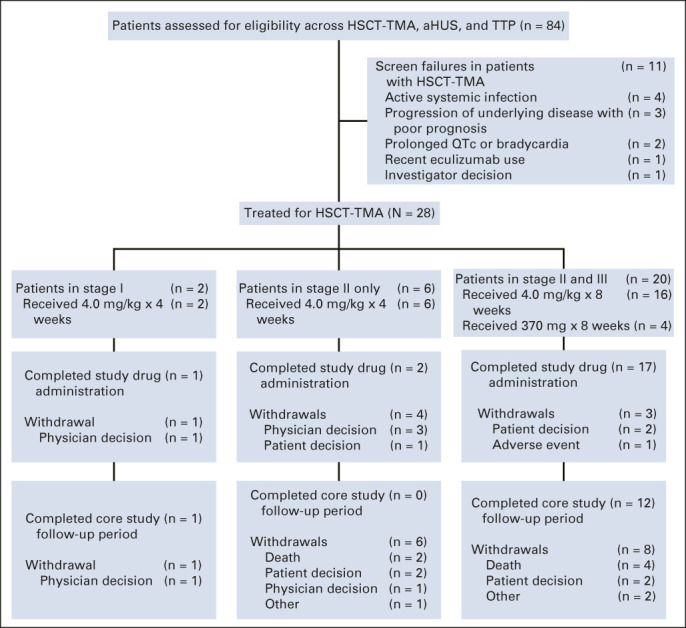
Flow diagram. Patient disposition during the core study period. Completion of study drug administration was defined by the assigned treatment (ie, eight once-weekly doses in stage III). aHUS, atypical hemolytic uremic syndrome; HSCT-TMA, hematopoietic stem-cell transplantation–associated thrombotic microangiopathy; QTc, corrected QT interval; TTP, thrombotic thrombocytopenic purpura.
