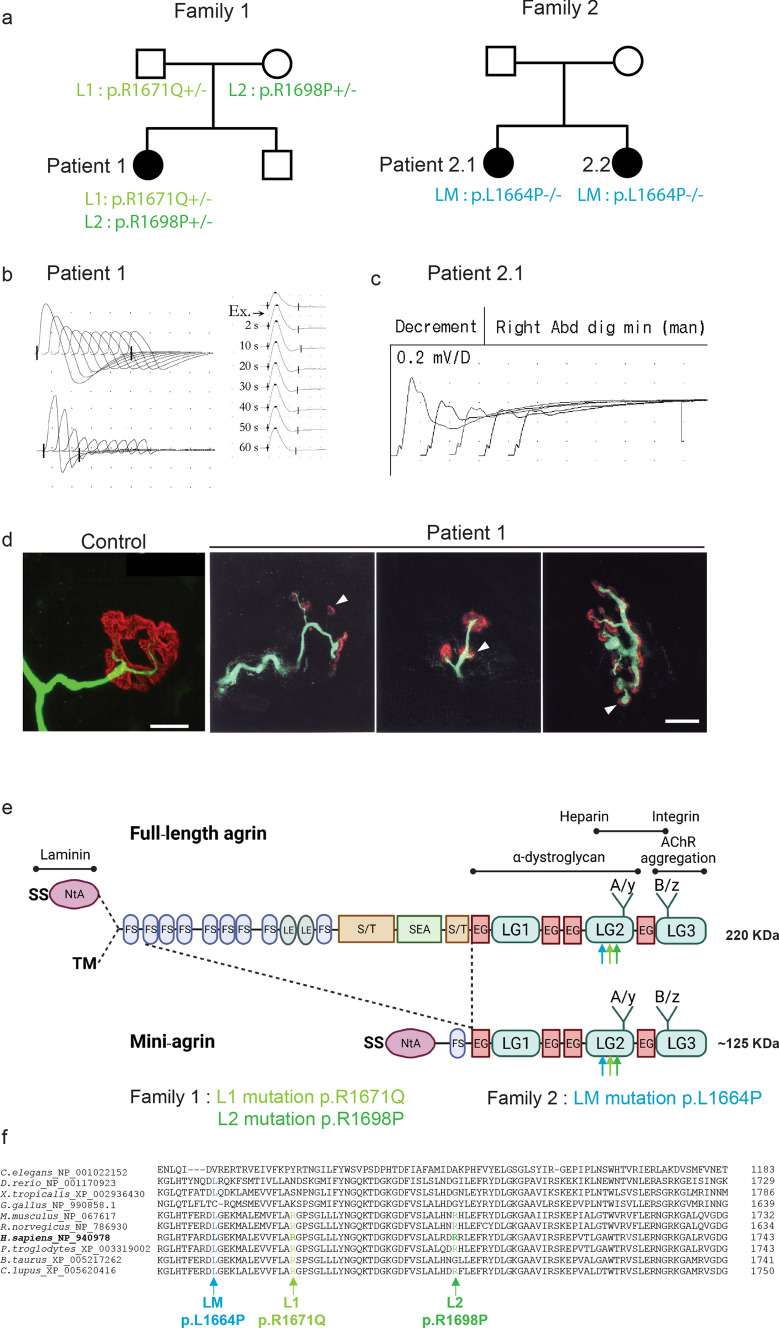
Fig. 1.
Mutations in AGRN Gene Causing CMS. a Family 1 and 2 pedigrees. b, c EMG reveals obvious decremental response of the compound muscle-fiber action potential (CMAPs) in Patient 1 and Patient 2.1. d Whole-mount preparations of muscle biopsy stained with α-bungarotoxin in red and stained for axon terminals with neurofilament in green. In control, the axonal branch classically ends as a fork and innervates a well-defined synaptic structure. In Patient 1, the neurofilament staining showed frequent presynaptic sprouting (arrowhead). Scale 10 µm. e Domain organization of agrin and recombinant mini-agrin used in this study and position of the L1, L2 and LM mutations. Agrin is a mosaic protein composed of the following domains: SS, signal peptide; NtA, N-terminal laminin-binding domain, red; TM, trans-membrane domain; FS, follistatin-like, blue; LE, laminin EGF-like, orange; S/T, serine/threonine-rich region, yellow; SEA, Sea urchin sperm protein, Enterokinase and Agrin motif, purple; EG, EGF-like, red; LG, laminin-globular-like, green; LG2 domain interacts with α-Dystroglycan and integrins; Sites of alternative splicing: SS-NtA or TM at the 5’end, A/y in the LG2 domain, B/z in the LG3 domain. f Multiple alignments of amino acid sequences in the agrin LG2 domains (residues 1949–1743 from H. sapiens_NP_940978) show the positions of L1, L2 and LM mutations