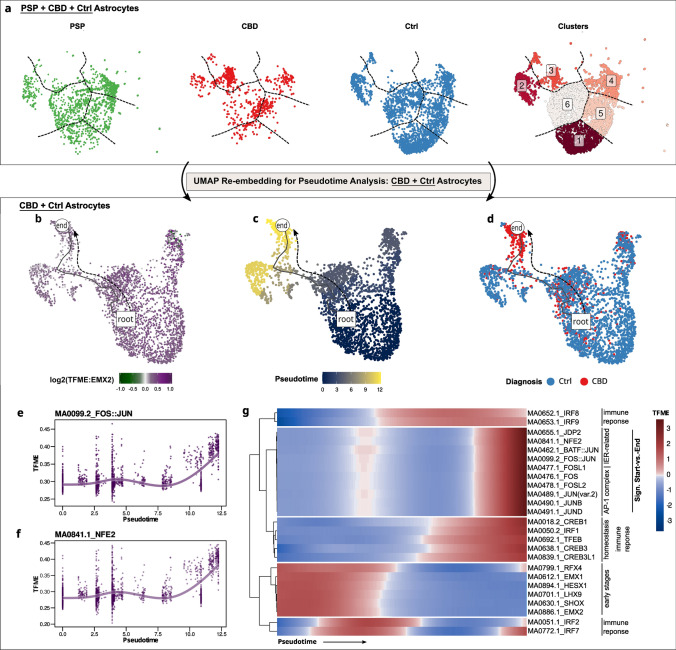
Fig. 4.
CBD astrocytes acquire an epigenetic state of stress response and neuroinflammation. a All PSP-, CBD-, and Ctrl-derived astrocytes re-embedded in UMAP, stratified by group entity (first, second, third panel), and depicted after k-means clustering in a merged UMAP (fourth panel). One cluster (#3) is specific for CBD astrocytes. Color code indicates group entity or cluster assignments in the first three or the fourth panel, respectively. Dashed lines delineate cluster borders and are transferred to the group-wise depictions. b–d Exclusively CBD- and Ctrl-derived astrocytes re-embedded in UMAP. A pseudotime trajectory leads from a non-specific Ast pool towards a CBD-enriched population. Color code indicates EMX2 TFME (b), pseudotime (c, dimensionless), or group entity (d). The black line indicates the pseudotemporal trajectory from the root towards the end cell. e, f Generative additive model non-linear fits of TFME values over pseudotime of the FOS-JUN (e) or NFE2 (f) motifs indicate parallel increments during the astrocytic transition towards a CBD-state. g Pseudotime heatmap displaying the TFME values of significantly altered TFMs in the start-vs.-end comparison (Wald-testing, BH-adjusted p < .05), as well as markers of early astrocytic development or immune regulation. Biological pathway associations are given on the right. TFME of astrocytic early-stage TFs is gradually decreasing, while immunologically relevant and AP-1 complex-related TFs gain in motif enrichment. IER immediate-early response, TFME transcription factor motif enrichment, Sign significant