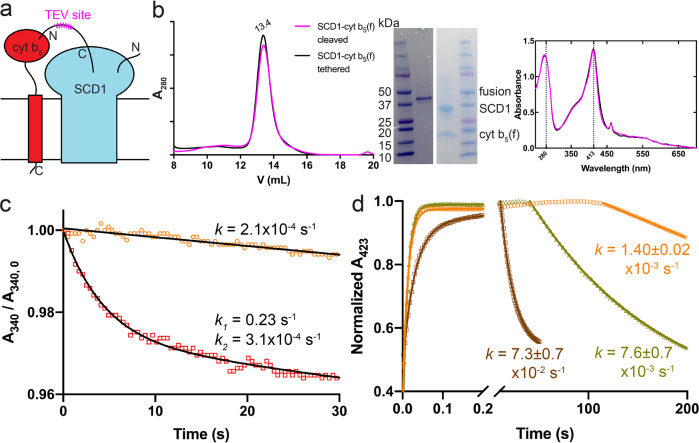
Fig. 4. SCD1 and full-length cyt b5 can form a stable complex with faster electron transfer kinetics.
a Schematic diagram of SCD1-cyt b5 fusion constructs. b SEC profile (left), SDS-PAGE image (inset), and UV-Vis spectra (right) of the linker-cleaved (magenta) and tethered (black) fusion of SCD1-cyt b5. Almost identical SEC profile and optical spectra suggest the stable assembly between SCD1 and cyt b5. c Biphasic kinetics of NADH consumption by b5R with SCD1-cyt b5 fusion in the presence of substrate stearoyl-CoA (red) compared to the slow linear decrease of NADH in the absence of substrate (orange). The absorbance of NADH at 340 nm (A340) was measured and the y axis is the normalized A340 against the initial values (A340, 0). Excess NADH was used in the measurements. d The accelerated electron transfer between reduced cyt b5 and SCD1 in the SCD1-cyt b5 complex (brown) compared to that between the individual cyt b5 and SCD1 (yellow) and auto-oxidation of cyt b5 (orange). The Soret absorbance of reduced cyt b5 at 423 nm was monitored. One molar equivalent of NADH was added, which resulted in the initial rising phases of the fast electron transfer to cyt b5 via b5R. Rate constants (k) are denoted as mean ± SEM calculated from three independent repeats (n = 3).