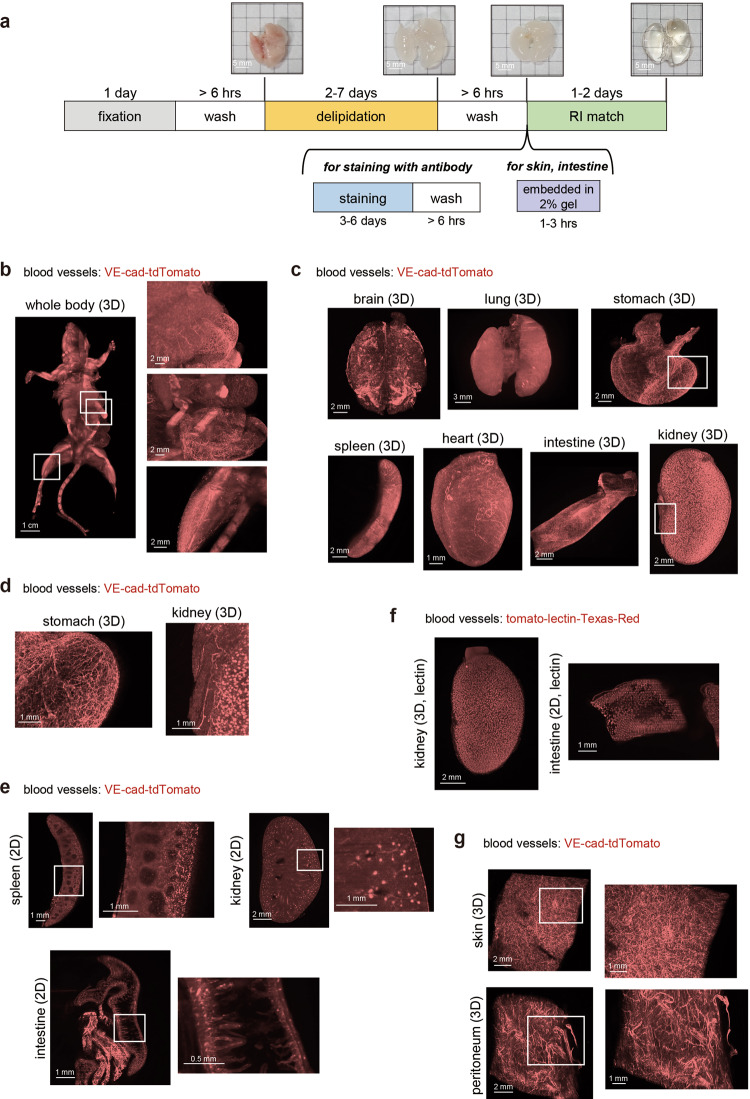
Fig. 1. Whole-body and whole-organ 3D imaging of mouse blood vessels.
a Whole-body/organ tissue-clearing protocol with CUBIC reagents. Mice were sacrificed and perfused with 4% PFA. The fixed samples were subjected to CUBIC procedures. The basic protocol consists of fixation, delipidation with CUBIC-L, and RI adjustment with CUBIC-R (N). For 3D immunostaining, staining with washing is inserted before RI adjustment. Samples from the skin and peritoneum were embedded into 2% gelatin before RI adjustment. The images of the transparent samples are captured using light sheet fluorescent microscopy (LSFM). The images of the lung in each step are shown. b The 3D-reconstituted (3D) body image of a VE-cad-tdTomato mouse (female, 4 months). Before CUBIC procedures, tamoxifen (Tx) was injected (i.p.) for the induction of reporter. The enlarged images of the abdominal organs (liver, stomach, intestine and leg) are shown in the right panels. c The 3D images of the whole organs, including brain, lung, stomach, spleen, heart, intestine, and kidney in VE-cad-tdTomato mice (2–4 months) (Z; 10 μm step, digital zoom; brain, lung: 1.25, stomach, spleen, and kidney: 1.6, heart: 2.5, intestine: 2.0). d The enlarged 3D images (white insets) (kidney and stomach) of (c). e The 2D (XY) images of the spleen, kidney, and intestine. White insets are magnified next to each image. f The 3D image of the kidney and 2D image of the intestine in tomato-lectin-injected mice (C57BL/6J, 5w). The mouse was sacrificed 5 min after inoculation of Texas-Red conjugated with tomato-lectin (Z; 10 μm step, digital zoom; kidney: 2.0, intestine: 1.6). g The 3D images of the blood vessels in the skin and peritoneum embedded in gelatin. The skin and peritoneum samples from a VE-cad-tdTomato mouse (3 months) were embedded in 2% gel before RI adjustment. White insets are magnified next to each image (Z; 10 μm step, digital zoom; 2.0).